A Narrative Review on the FSTL-1 Protein and its Current Known Impact on Cardiovascular Ischaemic Disease
DOI:
https://doi.org/10.5195/ijms.2024.2297Keywords:
Myocardial Ischemia, Coronary Artery Disease, Follistatin-Related Proteins, Follistatin-Related Protein 1 , FSTL-1 Protein, Cardiovascular Regeneration, Myocardial Infarction, Ischaemic Heart Disease, Cardiac Stem Cells, Anti-Inflammatory Properties, Angiogenesis, Fibrosis Reduction, AKT and ERK Pathways, Therapeutic TargetAbstract
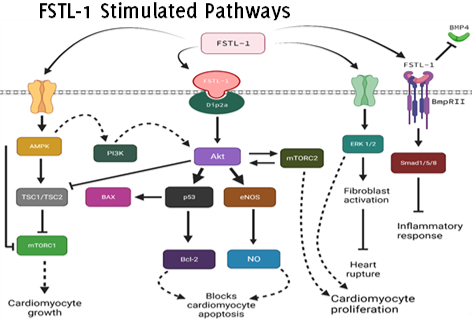
This narrative review investigates the potential therapeutic role of FSTL-1 in addressing severe cardiac issues following myocardial infarctions (MI). Despite advances in modern medicine, MI persist as a leading global cause of death, with stem cell therapy falling short of expectations since the early 2000s. In contrast, FSTL-1, an emerging bone morphogenetic protein, demonstrates promise based on successful studies. We conducted a qualitative narrative synthesis of studies published in PubMed, Scopus, and Web of Science between January 2000 and May 2022. This research explores the intricate scientific aspects of FSTL-1's contribution to myocardial regeneration, utilizing a chronological approach to trace its progression from biological pathways to broader scenarios. It examines the mechanisms regulated by FSTL-1 and its effects on cardiac tissue and cells, highlighting its potential as a therapeutic agent emphasizing its multifaceted role in cardiac regeneration. By deepening our comprehension of FSTL-1, this study significantly contributes to knowledge advancement, offering insights into its role in addressing severe cardiac issues post-MI. By consolidating current knowledge and proposing new avenues for investigation, this work offers valuable insights into FSTL-1's significance in advancing cardiovascular health and post-MI recovery.
References
Rørholm Pedersen L, Prescott E, Kerins M. ESC prevention of CVD Programme: Epidemiology of IHD. ESC Prevention of CVD Programme: Epidemiology of IHD. 2017;12(6):494-502.
du Pré BC, Doevendans PA, van Laake LW. Stem cells for cardiac repair: an introduction. J Geriatr Cardiol. 2020;17(2):1-5.
Daneshi N, Bahmaie N, Esmaeilzadeh A. Cell-Free Treatments: A New Generation of Targeted Therapies for Treatment of Ischemic Heart Disease. 2021.
Kim Y, Zharkinbekov Z, Sarsenova M, Yeltay G, Saparov A. Recent Advances in Gene Therapy for Cardiac Tissue Regeneration. Int J Mol Sci. 2022;23(3):1025.
Vasu S, Zhou J, Chen J, Johnston PV, Kim DH. Biomaterials-based Approaches for Cardiac Regeneration. Korean Circ J. 2020;50(6):498-506.
Peters MC, Di Martino S, Boelens T, Qin J, van Mil A, Doevendans PA, et al. Follistatin-like 1 promotes proliferation of matured human hypoxic iPSC-cardiomyocytes and is secreted by cardiac fibroblasts. Mol Ther Methods Clin Dev. 2019;14:93-105.
Ojha N, Dhamoon AS. Myocardial Infarction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108(11):1395-403.
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilises two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037-47.
Santini MP, Tsao L, Monassier L, Theodoropoulos C, Carter J, Lara-Pezzi E, et al. Enhancing repair of the mammalian heart. Circ Res. 2007;100(11):1733-6.
Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990;111(2):743-55.
Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20(3):334-47.
Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularisation in ischemic tissue through a nitric-oxide synthase-dependent mechanism. 2008.
Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation. 2012;126(14):1728-38.
Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011;90(2):224-33.
Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel E, et al. Follistatin-Like 1 Is an Akt-Regulated Cardioprotective Factor That Is Secreted by the Heart. Circulation. 2008;117(24):3099-108.
Xi Y, Gong D-W, Tian Z. FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial Infarction Rats. Sci Rep. 2016;6:32445.
Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86(8):927-34.
Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19(23):6341-50.
Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77(3):463-70.
Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13(12):1467-75.
Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, et al. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture. EMBO Mol Med. 2016;8(8):949-66.
Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95(8):773-9.
Geng Y, Dong Y, Yu M, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108(17):7058-63.
Formigli L, Manneschi LI, Nediani C, Marcelli E, Fratini G, Orlandini SZ, et al. Are macrophages involved in early myocardial reperfusion injury? Ann Thorac Surg. 2000;70(6):1969-74.
Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World J Cardiol. 2010;2(11):345-52.
Pachori AS, Custer L, Hansen D, Clapp S, Kemppa E, Klingensmith J. Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent signaling pathway. J Mol Cell Cardiol. 2010;48(6):1255-62.
Formigli L, Manneschi LI, Nediani C, Marcelli E, Fratini G, Orlandini SZ, et al. Are macrophages involved in early myocardial reperfusion injury? Ann Thorac Surg. 2000;70(6):1969-74.
Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World J Cardiol. 2010;2(11):345-52.
Shen H, Cui G, Li Y, et al. Follistatin-like 1 protects mesenchymal stem cells from hypoxic damage and enhances their therapeutic efficacy in a mouse myocardial infarction model. Stem Cell Res. 2012;9(2):140-52.
Van Wijk B, Gunst QD, Moorman AFM, et al. Cardiac Regeneration from Activated Epicardium. PLoS One. 2012;7(10):e45626.
Shimano M, Ouchi N, Nakamura K, Van Wijk B, Ohashi K, Asaumi Y, et al. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci U S A. 2011;108(29):11727-32.
Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, Zhao M, Maruyama S, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479-85.
Chen W, Xia J, Hu P, Zhou F, Chen Y, Wu J, et al. Follistatin-like 1 protects cardiomyoblasts from injury induced by sodium nitroprusside through modulating Akt and Smad1/5/9 signaling. Biochem Biophys Res Commun. 2015;460(2):259-65.
Pérez-Pomares JM, De La Pompa JL. Signaling During Epicardium and Coronary Vessel Development. Circ Res. 2011;109(12):1421-35.
Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827-42.
Hambrock HO, Kaufmann B, Muller S, Hanisch FG, Nose K, Paulsson M, et al. Structural characterisation of TSC-36/Flik: analysis of two charge isoforms. J Biol Chem. 2004;279(11):11727-35.
Rossdeutsch A, Smart N, Dubé KN, Turner M, Riley PR. Essential role for thymosin β4 in regulating vascular smooth muscle cell development and vessel wall stability. Circ Res. 2012;111(8):e89-e102.
Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, et al. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture. EMBO Mol Med. 2016;8(8):949-66.
Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474(7353):640-4.
Shrivastava S, Srivastava D, Olson EN, DiMaio JM, Bock-Marquette I. Thymosin beta4 and cardiac repair. Ann N Y Acad Sci. 2010;1194:87-96.
Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109-13.
Cao J, Poss KD. The epicardium as a hub for heart regeneration. Nat Rev Cardiol. 2018;15(10):631-47.
Görgens SW, Raschke S, Holven KB, Jensen J, Eckardt K, Eckel J. Regulation of follistatin-like protein 1 expression and secretion in primary human skeletal muscle cells. Arch Physiol Biochem. 2013;119(1):1-8.
Miyabe M, Ohashi K, Shibata R, Uemura Y, Ogura Y, Yuasa D, et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res. 2011;91(4):516-24.
Xi Y, Gong DW, Tian Z. FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial Infarction Rats. Sci Rep. 2015;5:15524.
Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, et al. CTRP9 Protein Protects against Myocardial Injury following Ischemia-Reperfusion through AMP-activated Protein Kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287(22):18965-73.
Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther. 2017;8:242.
Lu L, Ma J, Liu Y, Shao Y, Xiong X, Duan W, et al. FSTL1-USP10-Notch1 Signaling Axis Protects Against Cardiac Dysfunction Through Inhibition of Myocardial Fibrosis in Diabetic Mice. Front Cell Dev Biol. 2020;8:192.
Published
How to Cite
License
Copyright (c) 2024 José Rodrigues Gomes, Mário Santos

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- The Author retains copyright in the Work, where the term “Work” shall include all digital objects that may result in subsequent electronic publication or distribution.
- Upon acceptance of the Work, the author shall grant to the Publisher the right of first publication of the Work.
- The Author shall grant to the Publisher and its agents the nonexclusive perpetual right and license to publish, archive, and make accessible the Work in whole or in part in all forms of media now or hereafter known under a Creative Commons Attribution 4.0 International License or its equivalent, which, for the avoidance of doubt, allows others to copy, distribute, and transmit the Work under the following conditions:
- Attribution—other users must attribute the Work in the manner specified by the author as indicated on the journal Web site; with the understanding that the above condition can be waived with permission from the Author and that where the Work or any of its elements is in the public domain under applicable law, that status is in no way affected by the license.
- The Author is able to enter into separate, additional contractual arrangements for the nonexclusive distribution of the journal's published version of the Work (e.g., post it to an institutional repository or publish it in a book), as long as there is provided in the document an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post online a prepublication manuscript (but not the Publisher’s final formatted PDF version of the Work) in institutional repositories or on their Websites prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work. Any such posting made before acceptance and publication of the Work shall be updated upon publication to include a reference to the Publisher-assigned DOI (Digital Object Identifier) and a link to the online abstract for the final published Work in the Journal.
- Upon Publisher’s request, the Author agrees to furnish promptly to Publisher, at the Author’s own expense, written evidence of the permissions, licenses, and consents for use of third-party material included within the Work, except as determined by Publisher to be covered by the principles of Fair Use.
- The Author represents and warrants that:
- the Work is the Author’s original work;
- the Author has not transferred, and will not transfer, exclusive rights in the Work to any third party;
- the Work is not pending review or under consideration by another publisher;
- the Work has not previously been published;
- the Work contains no misrepresentation or infringement of the Work or property of other authors or third parties; and
- the Work contains no libel, invasion of privacy, or other unlawful matter.
- The Author agrees to indemnify and hold Publisher harmless from the Author’s breach of the representations and warranties contained in Paragraph 6 above, as well as any claim or proceeding relating to Publisher’s use and publication of any content contained in the Work, including third-party content.
Enforcement of copyright
The IJMS takes the protection of copyright very seriously.
If the IJMS discovers that you have used its copyright materials in contravention of the license above, the IJMS may bring legal proceedings against you seeking reparation and an injunction to stop you using those materials. You could also be ordered to pay legal costs.
If you become aware of any use of the IJMS' copyright materials that contravenes or may contravene the license above, please report this by email to contact@ijms.org
Infringing material
If you become aware of any material on the website that you believe infringes your or any other person's copyright, please report this by email to contact@ijms.org







