A Descriptive Analysis of the Use of Various Therapeutics in a Cohort of COVID-19 Patients and the Influence of Media Coverage
DOI:
https://doi.org/10.5195/ijms.2024.2125Keywords:
COVID-19, SARS-CoV-2, Standard of Care, COVID-19 Treatments, Antiviral Therapy, Media Influence, Remdesivir, Hydroxychloroquine, Hospitalized Patients, Retrospective Study, Clinical Guidelines, Therapeutic Variation, Texas HealthcareAbstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) impacted the healthcare system immensely throughout 2020-2022. Treatment practices varied in Texas, as guidelines were in flux. As a result, a variety of therapeutics were used. Many verified medications with scientific basis were trialed, while others were implemented despite a lack of scientific consensus. This study aimed to identify how practice patterns to treat and manage COVID-19 changed over time in a cohort of patients in the University of Texas Medical Branch hospital system.
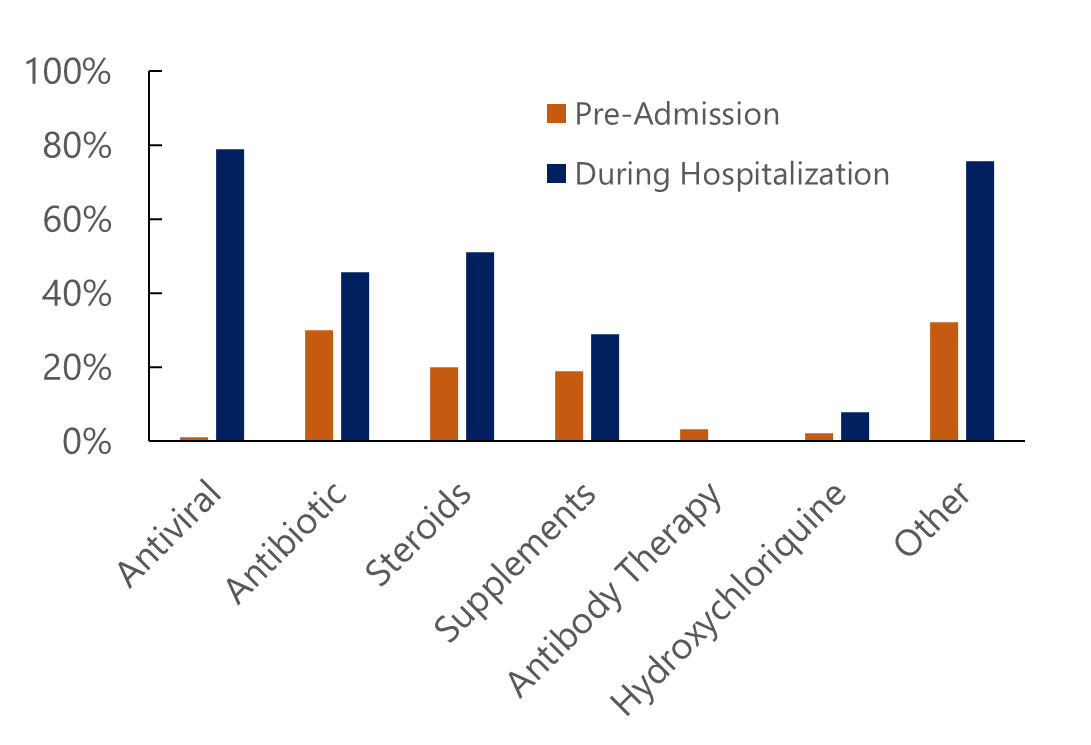
Methods: Ninety participants with a COVID-19 diagnosis were included in the analysis for this study. Data was collected by a retrospective chart review, and included medications administered before and during current admission. Medications were categorized as: antiviral, antibiotic, steroid, supplement, antibody, hydroxychloroquine, and others.
Results: Differences in therapeutic use were identified based on hospitalization status (outpatient or inpatient) and month admitted. The largest difference in the antiviral remdesivir (78%), requiring intravenous administration for up to ten days. In the outpatient setting, antibiotics, primarily azithromycin, were quite common. Additionally, month-to-month variation in steroid use and antibiotic use was observed.
Conclusion: This study shows that adapting medical guidelines and strong media coverage played a role in the clinical management of COVID-19 patients. At times, some ineffective medications were prescribed such as hydroxychloroquine. Medical leaders and news coverage should collaborate closely in future public health emergencies to prevent the prescription of ultimately ineffective and potentially hazardous treatments.
References
Monegro AF, Muppidi V, Regunath H. Hospital-Acquired Infections. StatPearls. Treasure Island (FL)2023.
Revelas A. Healthcare - associated infections: A public health problem. Niger Med J. 2012;53(2):59-64.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020.
Recovery Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693-704.
Recovery Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383(21):2030-40.
Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment [press release]. 05/01/2020 2020.
Bhimraj A MR, Shumaker AH, Baden L, Cheng VC, Edwards KM, Gallagher JC, Gandhi RT, Muller WJ, Nakamura MM, O'Horo JC, Shafer RW, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19: Infectious Diseases Society of America; 2022 [Version 10.0.0:[Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health 2022 [Available from: https://www.covid19treatmentguidelines.nih.gov/.
World Health Organization Communicable Diseases Emergencies Preparedness Emerging Diseases Clinical Assessment and Response Network Guidelines Review Committee. Therapeutics and COVID-19: Living guideline 2022 [Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5.
Bradley MC, Perez-Vilar S, Chillarige Y, Dong D, Martinez AI, Weckstein AR, et al. Systemic Corticosteroid Use for COVID-19 in US Outpatient Settings From April 2020 to August 2021. JAMA. 2022;327(20):2015-8.
Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55(6):105982.
Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020 [press release]. 03/30/2020 2020.
Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine [press release]. 06/15/2020 2020.
Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb Res. 2020;194:101-15.
Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 [press release]. 02/09/2021 2021.
Parra-Lara LG, Martinez-Arboleda JJ, Rosso F. Azithromycin and SARS-CoV-2 infection: Where we are now and where we are going. J Glob Antimicrob Resist. 2020;22:680-4.
Oldenburg CE, Pinsky BA, Brogdon J, Chen C, Ruder K, Zhong L, et al. Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA. 2021;326(6):490-8.
Diyya ASM, Thomas NV. Multiple Micronutrient Supplementation: As a Supportive Therapy in the Treatment of COVID-19. Biomed Res Int. 2022;2022:3323825.
Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, et al. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020;12(12).
FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising COVID–19 Treatment, Another Achievement in Administration’s Fight Against Pandemic [press release]. 08/23/2020 2020.
FDA In Brief: FDA Updates Emergency Use Authorization for COVID-19 Convalescent Plasma to Reflect New Data [press release]. 02/04/2021 2021.
Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, Tzanninis IG, et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21(2):167-79.
Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, et al. Early Convalescent Plasma for High-Risk Outpatients with Covid-19. N Engl J Med. 2021;385(21):1951-60.
Recovery Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397(10289):2049-59.
Potter GE, Bonnett T, Rubenstein K, Lindholm DA, Rapaka RR, Doernberg SB, et al. Temporal Improvements in COVID-19 Outcomes for Hospitalized Adults: A Post Hoc Observational Study of Remdesivir Group Participants in the Adaptive COVID-19 Treatment Trial. Ann Intern Med. 2022;175(12):1716-27.
Cohen J. Update: Here's what is known about Trump's COVID-19 treatment. 2020 10/05/2020.
Cathey L. Timeline: Tracking Trump alongside scientific developments on hydroxychloroquine 2020 [Available from: https://abcnews.go.com/Health/timeline-tracking-trump-alongside-scientific-developments-hydroxychloroquine/story?id=72170553.
McCarthy T GJ. Trump is taking hydroxychloroquine, White House confirms 2020 [Available from: https://www.theguardian.com/us-news/2020/may/19/trump-hydroxychloroquine-covid-19-white-house.
Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023;22(6):449-75.
Cokljat M, Cruz CV, Carrara VI, Puttaraksa K, Capriglioni C, Insaurralde SM, et al. Comparison of WHO versus national COVID-19 therapeutic guidelines across the world: not exactly a perfect match. BMJ Glob Health. 2024;9(4).
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2024 Alfred A. Mathew, Barbara Mensah, John C. Cravero, David C. Moffatt, Roshan Dongre, Thao K. Giang, Samantha C. Olvera, Susan L.F. McLellan, Corri B. Levine

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- The Author retains copyright in the Work, where the term “Work” shall include all digital objects that may result in subsequent electronic publication or distribution.
- Upon acceptance of the Work, the author shall grant to the Publisher the right of first publication of the Work.
- The Author shall grant to the Publisher and its agents the nonexclusive perpetual right and license to publish, archive, and make accessible the Work in whole or in part in all forms of media now or hereafter known under a Creative Commons Attribution 4.0 International License or its equivalent, which, for the avoidance of doubt, allows others to copy, distribute, and transmit the Work under the following conditions:
- Attribution—other users must attribute the Work in the manner specified by the author as indicated on the journal Web site; with the understanding that the above condition can be waived with permission from the Author and that where the Work or any of its elements is in the public domain under applicable law, that status is in no way affected by the license.
- The Author is able to enter into separate, additional contractual arrangements for the nonexclusive distribution of the journal's published version of the Work (e.g., post it to an institutional repository or publish it in a book), as long as there is provided in the document an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post online a prepublication manuscript (but not the Publisher’s final formatted PDF version of the Work) in institutional repositories or on their Websites prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work. Any such posting made before acceptance and publication of the Work shall be updated upon publication to include a reference to the Publisher-assigned DOI (Digital Object Identifier) and a link to the online abstract for the final published Work in the Journal.
- Upon Publisher’s request, the Author agrees to furnish promptly to Publisher, at the Author’s own expense, written evidence of the permissions, licenses, and consents for use of third-party material included within the Work, except as determined by Publisher to be covered by the principles of Fair Use.
- The Author represents and warrants that:
- the Work is the Author’s original work;
- the Author has not transferred, and will not transfer, exclusive rights in the Work to any third party;
- the Work is not pending review or under consideration by another publisher;
- the Work has not previously been published;
- the Work contains no misrepresentation or infringement of the Work or property of other authors or third parties; and
- the Work contains no libel, invasion of privacy, or other unlawful matter.
- The Author agrees to indemnify and hold Publisher harmless from the Author’s breach of the representations and warranties contained in Paragraph 6 above, as well as any claim or proceeding relating to Publisher’s use and publication of any content contained in the Work, including third-party content.
Enforcement of copyright
The IJMS takes the protection of copyright very seriously.
If the IJMS discovers that you have used its copyright materials in contravention of the license above, the IJMS may bring legal proceedings against you seeking reparation and an injunction to stop you using those materials. You could also be ordered to pay legal costs.
If you become aware of any use of the IJMS' copyright materials that contravenes or may contravene the license above, please report this by email to contact@ijms.org
Infringing material
If you become aware of any material on the website that you believe infringes your or any other person's copyright, please report this by email to contact@ijms.org







