Original Article
Scatterplot Variations Seen in Malaria Using Automated Hematological Analyzers: A
Series of Ten Cases
Ronit Juthani1, Tavish Gupta2, Debdatta Basu3
doi: http://dx.doi.org/10.5195/ijms.2021.866
Volume 9, Number 1: 21-24
Received 03 12 2020:
Rev-request 06 02 2021:
Rev-request 18 04 2021:
Rev-recd 07 02 2021:
Rev-recd 18 04 2021:
Accepted 18 04 2021
ABSTRACT
Background:
Malaria is a major health problem in India. Complete blood count and peripheral blood
smear (PBS) are important for its diagnosis. Interobserver variation makes PBS fallible.
Rapid diagnostic tests cannot detect low parasitemia and mixed infections. Scatterplot
from automated analyzers have shown variations previously which might be exploited.
Methods:
This descriptive study was conducted between July and August 2018. Scatterplot patterns
of ten samples of confirmed malaria and 100 control samples were derived using automated
hematology analyzers All other infections were ruled out by relevant culture and serology.
Each malarial scatterplot was compared with the control pattern for abnormalities
and their frequency was noted.
Results:
All ten samples belonged to the Plasmodium vivax species. Abnormalities detected included split in neutrophilic region, eosinophil-neutrophil
merge, neutrophil graying, lymphopenia, ghost red blood cells eosinophil split, reactive
lymphocytes, monocytosis, pseudoeosinophilia and neutrophilic leukocytosis.
Conclusion:
Variations in scatterplot patterns are seen in malaria and provide clues to the diagnosis
of malaria.
Keywords:
Hematologic Tests;
Diagnosis;
Malaria (Source: MeSH-NLM).
Introduction
Malaria is a major health care problem in India. In the World Malaria Report 2020
produced by the World Health Organization (WHO), India currently accounts for 3% of
the global malaria burden and contributes to 86% of total malaria cases in Southeast
Asia.1 Plasmodium falciparum and Plasmodium vivax are the dominant species responsible for the spread, with both being reported in
almost equal proportions in India and varying based on regions.
The primary investigations ordered in suspected malaria include a complete blood count
and peripheral blood smear (PBS) besides other serological and microbiological analyses.
While these investigations are admirable and help in identifying a large case load,
the true burden of the disease is estimated to be much higher than the above number.
PBS examination remains a tedious process which is time consuming and subjective,
based on the expertise of the examining person.2 Low detection levels, especially at low parasite levels, limits the accuracy of a
microscope. Expertise may bring about variations, with the most experienced microscope
users detecting numbers as low as 5 parasites/µL while the average user detects 50
parasites/µL.
Asymptomatic cases with low parasite numbers may thus be underestimated.3 As much as 25% of malaria cases may be missed by microscopy.4 Rapid diagnostic tests (RDT), on the other hand, are a poor choice in cases having
low density parasitemia and mixed infection with twin malaria species.5 Performance may also be affected by temperature and humidity variations which damage
the nitrocellulose membrane and bound monoclonal antibodies of RDT, thus affecting
its performance.6
Modern hematologic analyzers work largely on two principles: optical scatter which
measures the deviation in the pathway of light caused by the size and granularity
of the cell and electrical impedance which measures the change in electric current
caused by blood cells.7 In a study previously conducted by us, we have shown how acute febrile illnesses
caused by an infectious etiology have shown variations in scatterplot patterns obtained
from automated hematologic analyzers.8 In particular, numerous studies have been conducted showing cell abnormalities represented
in peculiar ways in the scatterplots of malarial patients, with species identification
also possible.9–11 In this study, we report on ten cases of malaria, confirmed on peripheral blood smear
examination which showed unique scatterplot patterns. We aim to highlight these new
features of scatterplot patterns associated with malaria infection.
Methods
This descriptive study was completed in the hematology laboratory of Jawaharlal Institute
of Postgraduate Medical Education and Research, Puducherry, India between July and
August 2018. K2 EDTA blood samples of cases with PBS and microbiologically confirmed
malaria were taken as study samples and samples with no history of fever and normal
white cell counts and differentials were taken as control. Since the study was performed
on blood samples taken as part of a routine investigation and patients remained anonymous,
ethics approval was waived by the Institute Ethics Committee. A total of ten cases
of malaria diagnosed during the time period along with 100 normal samples were studied
in the automated Sysmex XT2000i hematology analyzer. A simultaneous culture and serology
were done for the control samples to rule out any hidden infection which may cause
variation in scatterplot pattern.
In each case, 2 mL of EDTA venous blood was collected and analyzed by automated analyzer.
In each case the complete blood counts and the scatterplot patterns were studied.
Comparison of each scatterplot generated from these cases was done with the prototype
control pattern (Figure 1) and the abnormalities were noted. The PBS was stained by Leishman stain with 2 minutes
fixation and 15 minutes staining and studied in details for the morphology of the
blood cells and presence of the malarial parasites.
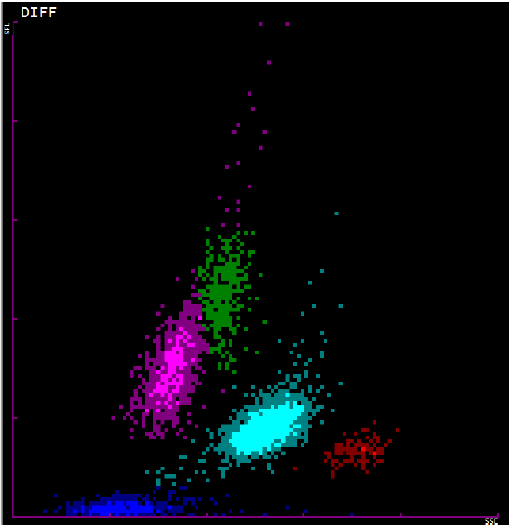
Figure 1.
Representative scatterplot showing the pattern of white blood cells in a peripheral
blood smear. The pink plot represents lymphocytes, green represents monocytes, light
blue represents neutrophils, red represents eosinophils, and red represents red blood
cells.
Results
A total of ten cases of malaria and 100 controls were collected and their scatterplots
generated using the Sysmex XT2000i analyzer. Out of the ten cases, five were taken
from one of our earlier studies on scatterplot and acute febrile illnesses conducted
around the same time.8 The representative control normal scatterplot pattern used is shown in Figure 1.
All cases of malaria were of the Plasmodium vivax species and were confirmed by both positive RDT and the presence of trophozoites
in the peripheral blood smear. The following findings were noted:
- A split in the neutrophil region was evident in 5 of the 10 samples. This was represented
by a change in shape of the light blue color from the normal ellipse to a double ellipse
joined at the ends.
- A merging of the eosinophilic region with the neutrophilic region was noted in 4 of
the 10 samples. This was represented by the blue and the red population merging together
without any space between them.
- Graying of the neutrophil area was seen in 2 out of the 10 samples. While we are considering
this as a separate entity, it may be considered a variant of the neutrophil-eosinophil
merge with the only difference being an inability to recognize neutrophils and eosinophils
as separate entities.
- Lymphopenia was noted in 4 of the 10 samples. This was indicated by:
- -Decrease in the area occupied by the pink color
- -Decrease in the intensity of the pink color
- Increased ghost red blood cells (RBC) were noted in 4 of the 10 samples. This was
experienced by an increase in the area or intensity of dark blue color which was greater
than two divisions on the x-axis.
- A split in the eosinophil population was noted in 3 of the 10 samples. This presented
in the scatterplot as two populations of red color separated by a band of black color
either in the x-axis or y-axis.
- Reactive lymphocyte populations were seen in 2 samples by a shower of pink cells which
were present over the green monocytic region.
- Besides pseudoeosinophilia, both monocytosis and neutrophilic leukocytosis were each
seen in 2 of the 10 samples.
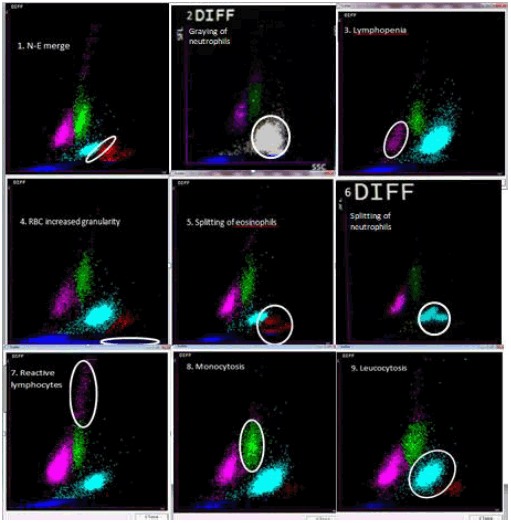
A composite image highlighting all the findings has been shown in Figure 2.
Figure 2.
Scatterplot variations and their interpretations:1. Neutrophil and eosinophil merge,
2. Graying of neutrophils, 3. Lymphopenia, 4. Increase in RBC granularity, 5. Splitting
of eosinophils, 6. Splitting of neutrophils, 7. Reactive lymphocytosis, 8. Monocytosis,
9. Leucocytosis.
Discussion
According to a WHO report in 2019, there were an estimated 409 000 deaths from malaria
globally.1 Hence, the diagnosis of malaria should be prompt and accurate so that treatment can
be started in a timely manner to avoid unnecessary complications. PBS examination
is often the first line of investigation in suspected cases of malaria and changes
in the scatterplot pattern, if carefully identified, it can help in identifying the
parasites earlier in the blood.
The key abnormalities found in our scatterplot analysis included neutrophil splitting,
eosinophil-neutrophil merge, graying of neutrophil region, lymphopenia, ghost RBC
increase and eosinophil split. Automated hematological analyzers are based on flow
cytometry. Special fluorescent dyes are used to stain nucleic acids. The channel lyses
RBCs along with platelets and binds the nucleic acid using a dye to give a fluorescence
proportionate to the nucleic acid content. The higher the percentage of nucleic acids,
the greater the intensity of scatterplot pattern.12
In all ten cases with scatterplot abnormalities, schizonts of Plasmodium vivax was seen in the peripheral smears, which was expected considering the low incidence
of Plasmodium falciparum in Pondicherry.13 Specific changes have been observed in Plasmodium vivax because of the presence of hemozoin pigments of the schizonts in the peripheral blood.
These changes have been more often seen in Plasmodium vivax than in Plasmodium falciparum, as schizonts are usually not seen in the peripheral blood in the latter.14,15 This may be a limitation of this detection method, as Plasmodium falciparum infections are missed by scatterplot investigations.
Earlier studies have found changes such as pseudoeosinophila and the graying of neutrophilic
areas as relevant findings, which make it pertinent to check for malarial parasites
in peripheral blood smear.16,17 We were evidently able to concur such findings in our study. Apart from that, we
were able to obtain unique findings of increased ghost RBC density, lymphopenia, and
a dual population of eosinophils represented by a split in the eosinophilic region,
which we found to be a good pointer of malaria. In 50% of our cases, neutrophil population
split was found. This could be a potentially strong indicator of malaria. Neutrophilic
merge with the eosinophilic region was also a unique finding, as was eosinophil split.
Reactive lymphocytosis and monocytosis are findings in a number of other illnesses
but should also arouse a strong suspicion of malaria if it can be correlated clinically
and epidemiologically as malaria.
A study conducted by Huh et al in South Korea extensively studied 144 cases of Plasmodium
vivax malaria and found a high incidence of pseudoeosinophilia characterized by a
difference in the eosinophil count detected by the analyzer and observed on the smear.18 This was found to be as high as 39%. In our study, we only detected two samples with
pseudoeosinophilia, showing that pseudoeosinophilia may not be a very sensitive finding.
This is in line with other Indian studies which have found spurious eosinophilia in
1.5-4% of the population.9,19 The same study by Huh et al also found similar findings of dual eosinophil and neutrophil
population; however, their incidence was very low compared with the pseudoeosinophil
population. In total, they found 52.10% scattergrams being abnormal, indicating their
importance in diagnosis.18 A follow up study conducted by Yoo et al in 2010 found abnormalities in 15.70% of
the scattergrams, despite finding the same incidence of pseudoeosinophilia.20 In fact, these authors hypothesized that pseudoeosinophilia, neutrophil clusters
and neutrophil-eosinophil merge were largely resulting from the hemozoin pigments
in neutrophils and shouldn't be considered a separate entity. While studies by Huh
et al. and Yoo et al. have found 52.10% and 15% of abnormal, in our study we found
all ten of our samples to report some kind of abnormal scatterplot, which was in line
with the Indian studies mentioned earlier that found 100% and 83.8% abnormal scatterplot
patterns.9,19 Thus, being aware of these scatterplot findings in the presence of clinical suspicion
may help in the early diagnosis and initiation of treatment in malaria. A limitation
of our study is the small sample size, yet it constitutes a sizeable number compared
to the annual incidence of malaria in Puducherry.
Conclusion
Scatterplot patterns in malaria have been reported with varying sensitivity and specificity.
We found abnormal scatterplot patterns in all the 10 cases of Plasmodium vivax malaria some of which have not been described before. These patterns should be kept
in mind by the pathologist or the laboratory personnel and should prompt a thorough
screening of the peripheral blood film to confirm for the parasites. When supplemented
with peripheral blood smear examination, they are an adjunct to the current diagnostic
modalities.
Acknowledgments:
None.
Conflict of Interest Statement & Funding:
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Author Contributions:
Conceptualization, Methodology, Project Administration, Resources, Supervision, Writing
– Review & Editing: DB. Data Curation, Investigation & Writing – Original Draft Preparation:
RJ, TV. Formal Analysis: RJ, TV, DB.
References
1.
World Malaria Report 2020, Geneva : World Health Organization 2020. Available from: https://apps.who.int/iris/rest/bitstreams/1321872/retrieve
2. Nema S, Verma AK, Bharti PK. Strengthening diagnosis is key to eliminating malaria in India. 2019 Dec;19(12):1277–8.
3. Ohrt C, Purnomo , Sutamihardia MA, Tang D, Kain KC. Impact of microscopy error on estimates of protective efficacy in malaria prevention
trials. J Infect Dis. 2002 Aug 15;186(4):540–6.
4. Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic
review and meta-analysis. J Infect Dis. 2009 Nov 15; 200(10):1509–17.
5. Krishna S, Bharti PK, Chandel HS, Ahmad A, Kumar R, Singh PP, et al. Detection of Mixed Infections with Plasmodium spp. by PCR, India, 2014. Emerg Infect Dis. 2015 Oct;21(10):1853–7.
6. Chiodini PL, Bowers K, Jorgensen P, Barnwell JW, Grady KK, Luchavez J, et al. The heat stability of plasmodium lactate dehydrogenase-based and histidine-rich protein
2-based malaria rapid diagnostic tests. Trans R Soc Trop Med Hyg 2007 Apr;101(4):331–37.
7. Green R, Wachsmann-Hogiu S. Development, history, and future of automated cell counters. Clin Lab Med. 2015 Mar;35(1):1–10.
8. Juthani R, Basu D. Use of scatterplot patterns derived from automated haematological analysers in the
diagnosis of acute febrile illnesses. J Clin Diagn Res. 2020 Feb;14(2): EC06–EC10
9. Singh A, Narang V, Sood N, Garg B, Gupta VK. Malaria diagnosis using automated analysers: A boon for hematopathologists in endemic
areas. J Clin Diagn Res. 2015 Oct;9(10):EC05–8.
10. Jain M, Gupta S, Jain J, Grover RK. Usefulness of automated cell counter in detection of malaria in a cancer set up-Our
experience. Indian J Pathol Microbiol. Oct-Dec 2012;55(4):467–73.
11. Sharma S, Sethi N, Pujani M, Kushwaha S, Sehgal S. Abnormal WBC scattergram: a clue to the diagnosis of malaria. Hematology. 2013 Mar;18(2):101–5.
12. Hill VL, Simpson VZ, Higgins JM, Hu Z, Stevens RA, Metcalf JA, et al. Evaluation of the performance of the sysmex XT-2000i hematology analyzer with whole
bloods stored at room temperature. Lab Med. 2009 Dec;40(12):709–18.
13. Dhiman S, Veer V, Dev V. Declining transmission of malaria in India: Accelerating towards elimination. In: Towards Malaria Elimination - A Leap Forward. InTech; 2018.
14. Campuzano-Zuluaga G, Hänscheid T, Grobusch MP. Automated haematology analysis to diagnose Malaria. Malar J. 2010 Nov 30;9:346.
15. Mubeen KH, Devadoss CW, Rangan RA, Gitanjali M, Prasanna S, Sunitha VP. Automated Hematology Analyzers in Diagnosis of Plasmodium vivax Malaria: an Adjunct
to Conventional Microscopy. Mediterr J Hematol Infect Dis. 2014 Jun 1; 6(1):e2014034.
16. Buoro S, Manenti B, Seghezzi M, Moioli V, Bagorria M, Callegaro A, et al. Abnormal scattergrams and cell population data generated by fully automated hematological
analyzers: New tools for screening malaria infection? Int J Lab Hematol. 2018 Jun;40(3):326–34.
17. Mohapatra S, Samantaray JC, Arulselvi S, Panda J, Munot K, Saxena R. Automated detection of malaria with haematology analyzer Sysmex XE-2100. Indian J Med Sci. 2011 Jan;65(1):26–31.
18. Huh J, Oh GY, Huh JW, Chae SL. Malaria detection with the Sysmex XE-2100 hematology analyzer using pseudoeosinophilia
and abnormal WBC scattergram. Ann Hematol. 2008 Sep;87(9):755–9.
19. Sharma S, Sethi N, Pujani M, Kushwaha S, Sehgal S. Abnormal WBC scattergram: a clue to the diagnosis of malaria. Hematology. 2013 Mar;18(2):101–5.
20. Yoo J-H, Song J, Lee K-A, Sun Y-K, Kim Y-A, Park TS, et al. Automated detection of malaria-associated pseudoeosinophilia and abnormal WBC scattergram
by the Sysmex XE-2100 hematology analyzer: a clinical study with 1,801 patients and
real-time quantitative PCR analysis in vivax malaria-endemic area. Am J Trop Med Hyg. 2010 Mar;82(3):412–4.
Ronit Juthani, 1 MBBS final year, Jawaharlal Institute of Postgraduate Medical Education & Research,
JIPMER, Government of India, Puducherry, India
Tavish Gupta, 2 Intern, JIPMER, Puducherry, India
Debdatta Basu, 3 Professor (Senior Scale) and Head, Department of Pathology, JIPMER, Puducherry, India
About the Author: Ronit Juthani is a Final Year Part 2 MBBS student of JIPMER, Puducherry. He is also
a recipient of the Indian Council Of Medical Research-Short Term Studentship (ICMR-STS)
award for 2017-2018 and the institutional Golden Jubilee Short Term Research Award
For Undergraduate Students (GJ-STRAUS) award for 2018-2019.
Correspondence: Ronit Juthani, Address: Jipmer Campus Rd, Gorimedu, Puducherry, 605006, India. Email:
ronitjuthani23@gmail.com
Editor: Paul Morgan
Student Editors: Nicole Katherine Conners & Lourdes Adriana Medina-Gaona
Copyeditor: Madeleine J. Cox
Proofreader: Sohaib Haseeb
Layout Editor: Francisco J. Bonilla-Escobar
Cite as: Juthani R, Gupta T, Basu D. Scatterplot Variations Seen in Malaria Using Automated Hematological Analyzers: A Series of Ten Cases. Int J Med Students. 2021 Jan-Apr;9(1):21-4.
Copyright © 2021 Ronit Juthani, Tavish Gupta, Debdatta Basu
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 9, NUMBER 1, April 2021

