Review
Comparative Review of Large Animal Models for Suitability of Proximal Aortic Endovascular
Repair
Abhishekh Srinivas1, Ming Yii2, Julian A. Smith3
doi: http://dx.doi.org/10.5195/ijms.2022.763
Volume 10, Number 2: 185-191
Received 12 12 2020:
Rev-request 04 01 2021:
Rev-request 09 04 2021:
Rev-request 06 08 2021:
Rev-recd 30 01 2021:
Rev-recd 09 06 2021:
Rev-recd 20 02 2022:
Accepted 21 02 2022
ABSTRACT
The advent of thoracic endovascular aortic repair (TEVAR) heralds a paradigm shift
in treating descending aortopathies. TEVAR is viewed as a potential option for ascending
aortic dissection (AD) repair. Currently, TEVAR's use in treating ascending aortopathies
remains limited. Appropriate animal models are urgently needed to improve our understanding
of the endovascular treatment of ascending ADs, also known as Stanford Type-A ADs.
This narrative review provides a current literature summary on the subject, including
the gross anatomical differences among adult porcine, ovine, and bovine species, compared
with those of their human counterparts, as well as specific valvular and coronary
vasculature measurement variances. An electronic search of Cochrane Library, PubMed,
and Ovid Medline databases from January 1965 to June 2020 was performed. The search
was limited to articles published in English. Twenty-three research papers were included
in this review. Our findings revealed that whereas macroscopic anatomy remains grossly
similar among these species, differences in valvular leaflet shape are present, with
porcine and ovine models possessing anatomic characteristics that are comparable to
their human counterparts. Inter-species differences concerning the anatomy of the
ascending aorta have not been extensively studied, highlighting a literature gap.
Conversely, multiple morphological studies have highlighted that porcine coronary
vasculature is similar to that of humans. In conclusion, both porcine and ovine species
are suitable as appropriate animal models for examining the feasibility of endovascular
stent-grafts for ascending ADs. However, given the similarities in coronary and aortic
valve anatomy with humans, porcine models are better suited for this purpose.
Keywords:
Aortic dissection;
Endovascular;
Ascending aorta;
Animal models (Source: MeSH-NLM).
Introduction
The use of non-human tissues in cardiothoracic medical research has markedly increased
over the last five decades as a solution to both the ethical dilemmas posed by using
human tissues and the lack of readily available human tissues for creating experimental
clinical models.1 One example of research involving such animal models is seen in a better understanding
treatment outcomes for acute aortic dissections (AD), a life-threatening pathology
that carries significant mortality rates of over 70% within one week of onset when
left untreated.2,3 Several classifications of ADs currently exist, but arguably perhaps, one of the
most commonly used is the Stanford classification system. This system categorizes
dissections based on the site of intimomedial tear as either Type-A, defined as any
AD involving the ascending aorta, or Type-B, which are ADs not involving the ascending
aorta (NB. This review focuses primarily on Type-A ADs).4
With few exceptions, managing acute Type-A ADs is touted as a surgical emergency.5,6 Given the aforementioned high rates of mortality otherwise, there are a few reasons
for not following through with operative treatment of Type-A Ads. The main cited reasons
are the presence of significant medical comorbidities that affect survival to one
year or less, as with very advanced age and frailty, advanced malignancies, or patient/family
wishes.7 The surgical intervention for Type-A ADs has markedly evolved over the years due
to the intertwined combination of technological improvements in equipment and a better
understanding of its natural history. Currently, open surgical repair (OSR) remains
the gold standard of care for this otherwise catastrophic condition.4,8 However, the advent of thoracic endovascular aortic repair (TEVAR) has heralded a
paradigm shift in treatment options for aortic diseases involving the descending aorta.
Therefore, TEVAR has been viewed as a potential option for ascending aortic repair,
and consequently Type-A AD surgical repair.9 As a result, selected patients who would otherwise be ineligible for OSR as indicated,
which typically comprise up to 20% of all individuals, would benefit from having the
opportunity of still receiving life-saving treatment in the form of minimally invasive
endovascular techniques.10
Various types of endovascular therapies, including branched stent-grafts and valve-carrying
conduits, are currently viewed as potential therapeutic options for Type-A ADs.10 However, the use of these novel therapeutic procedures within a clinical setting
remains limited, with isolated case reports and case series providing the bulk of
currently available literature on patient outcomes. Consequently, appropriate animal
models are urgently needed to improve our understanding of the endovascular treatment
of Type-A ADs.
While there is a wide amount of published research on the variances of cardiothoracic
anatomy in non-human species, no literature review synthesizes this information, highlighting
the accelerated need for one to be formulated. Consequently, this review article aims
to combat this issue by providing a summary of currently available information on
this topic, with a particular focus on determining which animal model amongst those
of adult porcine, ovine, or bovine species would be ideal for research pertaining
to endovascular treatment of Type-A ADs, relevant to the practicing surgeon. Three
broad sections shall be covered, beginning with a discussion on the macroscopic anatomical
differences between humans, porcines, ovines, and bovines. The review shall then focus
on specific aspects of cardiothoracic anatomy, explicating the valvular, aortic, and
coronary vasculature differences. Finally, the suitability of which animal would be
best for use as clinical experimental models, from a strictly anatomical standpoint
for bettering our understanding of Type-A AD treatment, shall be explored.
Methods
For this review two databases were used: Ovid Medline and PubMed. Within Ovid Medline,
since the term ‘Type A aortic dissection' is well known within medical literature
(as opposed to its verbatim analogue ‘Stanford Type A aortic dissection'), the search
string was commenced by initially mapping the keyword ‘Endovascular' with the MeSH
term ‘Type A aortic dissection'. This was followed by using the Boolean operator ‘AND'.
The keyword ‘models' was used, and finally, the Boolean operator ‘AND' was used to
combine all search strings. Twelve results were obtained from Ovid Medline. For this
review, search results were limited to the English language. Furthermore, within PubMed,
an advanced search was conducted using the search terms ‘endovascular', ‘aortic dissection',
and ‘animal model'. The search yielded 26 articles, which were then analyzed in conjunction
with previous results obtained through Ovid Medline. A flowchart of our search strategy
and study selection is detailed below.
Finally, images from the University of Minnesota Atlas of Human Cardiac Anatomy were
used with permission to obtain a better pictorial representation of the cardiothoracic
anatomical variations among the porcine, ovine, and bovine models.
Results
Anatomical Considerations for Endovascular Therapy of Type-A Dissections amongst Humans
Despite the advantages of thoracic endovascular aortic repair (TEVAR) use, including
the elimination of the need for perioperative cardiopulmonary bypass and the requirement
for a major operative incision, such as a sternotomy, there exist certain limitations
that prevent its routine use in the current treatment of Type-A ADs.4,11–13 Given the paucity of large-scale trials documenting its efficacy and long-term follow-up
of patients who receive this modality of treatment, there exists a literature gap
in describing the specific limitations of endovascular therapy for ascending aortic
pathologies. The anatomical constraints of this novel therapy have been scrutinized
and shall now be explored further.
One of the major challenges in successfully treating Type-A ADs with currently available
stent-grafts lies in the need to insert a straight device into a curved structure
(the aortic arch), which poses a high risk of developing an endoleak. In simplifying
landmarks within the complex anatomy of the aortic arch, the Ishimaru classification
is commonly used to categorize thoracic aortic ‘zones' for stent-grafts.14
With Ishimaru's zone classifications, it is essential to ensure a ‘safe' distance
between the proximal and distal landing zones to facilitate successful stent-graft
deployment and avoid catastrophic aortic rupture.3,15,16 However, this measurement remains dependent on the characteristics of the chosen
stent-graft and the surgeon's technical expertise. Consequently, although some variation
in what constitutes a 'safe' distance exists, a proposed criterion has been a length
of at least 20 mm between the two landing zones to avoid aortic rupture during graft
deployment.16
Furthermore, problems are also created by the entry dissection tear occurring proximally
within Zone 0 as illustrated in Figure 1, specifically proximal to the sinotubular junction. A tear occurring within this
region would fail to allow endograft deployment in a manner that would allow coronary
blood flow to be maintained.15 Occlusion of the coronary ostia by closed ends of the stent-graft would cause ischemia
of the myocardium, resulting in potentially irreversible damage.17,18 Additionally, those with Type-A ADs extending into the aortic valve would not be
suitable for endovascular treatment with conventional stent-grafts, a situation typically
observed in 10–20% of patients.15 At deployment, the tip of the device must cross the aortic valve, which could lead
to possible ventricular perforation. Although this would pose a barrier to treatment
with currently available stent-grafts, given that they possess a distal cone that
prevents their deployment too close to the aortic valve. A proposed method to combat
this has been suggested in the form of novel ‘valve-carrying conduits'.
Figure 1
Ishimaru Classification of Various Landing Zones of Proximal Aorta for Endovascular
Arch Repair.
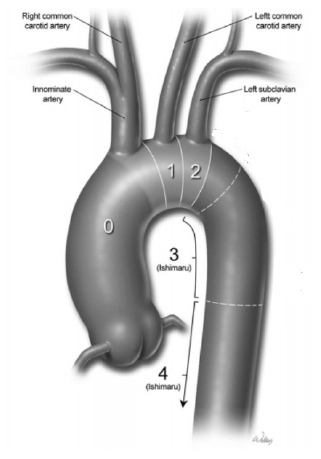
Legend: Reference: Zanotti G, Reece TB, Aftab M. Aortic Arch Pathology: Surgical Options
for the Aortic Arch Replacement. Cardiol Clin. 2017; 35(3):367-85. Printed with permission from Baylor College of Medicine.14
Thirdly, variations in the anatomy of the normal aorta may interfere with a wholly
endovascular modality of treatment for Type-A ADs. For instance, in patients who have
undergone prior coronary artery bypass surgery, the presence of coronary grafts arising
directly from the ascending aorta would present an increased risk of myocardial ischemia
during endograft deployment.15,16
Based on these caveats, it is evident that the anatomy of the ascending aorta, aortic
valve, and coronary vasculature are of particular significance in determining an appropriate
animal model for Type-A dissection research, which shall be addressed in the following
sub-section.
Introduction and General Cardiac Anatomy
Similar to humans, large mammals' holistic cardiac anatomy is analogous. Four cardiac
valves are present with similar structures comparable to most quadruped mammals. Whilst
human hearts can appear in various shapes, including elliptical, trapezoidal, and
‘valentine', porcine species tend to be valentine-shaped, while the ovine heart varies
from valentine to conical in shape, as illustrated in Figure 2.19
Figure 2
Plastinated Human (upper left), Ovine (upper right) and Porcine (bottom) Hearts.
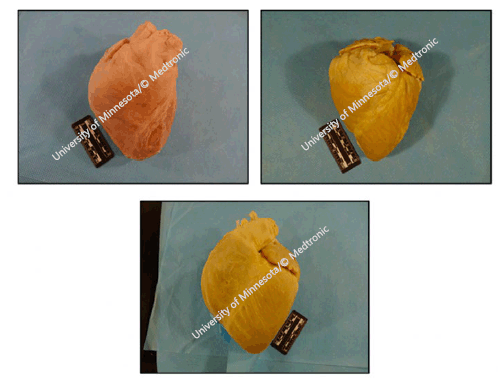
Legend: Reference: Atlas of Human Cardiac Anatomy, University of Minnesota/© Medtronic. Comparative
Anatomy of the Valves. Available from: http://www.vhlab.umn.edu/atlas/comparative-anatomy-tutorial/valves.shtml. Last updated Jan 14,2019; cited Jan 20,2020.19
Concerning the hearts of porcine and ovine species, the distance between the posteroinferior
base to apex, left lateral base to apex, and the coronary sinuses' length are significantly
greater than their human counterparts. Therefore, in conjunction with its larger size,
the average human heart maintains a larger organ-to-body weight ratio than both porcine
and ovine species. A similar scenario is visible in that of bovines, which possess
a nearly identical organ-to-body weight ratio to the ovine species.19
Valvular Anatomy
While the general cardiac anatomy of different hearts remains roughly similar, variations
in the four valves exist that distinguish among porcine, ovine, bovine, and human
species, despite certain structural similarities. Illustrated in Table 1, average aortic valve annulus diameters for humans are identical to those of their
porcine counterparts, with the ovine species possessing a slightly narrower annulus
on average. Conversely, bovine diameters are nearly 40% greater than their human counterparts,
possibly accounted for due to the increased cardiac output within this species.20
Table 1.
Mean Dimensions and Standard Deviations of Aortic Valve Measurement.
| Measurement (mm) |
Human |
Porcine |
Bovine |
Ovine |
| Annulus diameter of aortic valve (obturator diameter) |
26.4 ± 3.15 20 |
26.6 ± 1.84 20 |
33.7 ± 2.74 20 |
25.8 ± 1.29 20 |
| Leaflet depth |
Non-coronary cusp |
9.1 ± 1.66 20 |
8.9 ± 1.46 20 |
9.2 ± 1.58 20 |
7.4 ± 1.36 20 |
|
Right coronary cusp |
9.8 ± 2.21 20 |
10.2 ± 1.45 20 |
9.9 ± 1.21 20 |
7.6 ± 1.26 20 |
|
Left coronary cusp |
9.3 ± 1.24 20 |
8.6 ± 1.56 20 |
9.9 ± 0.96 20 |
7.8 ± 1.77 20 |
| Valvular commis-sure height |
Non-coronary cusp |
18.5 ± 1.96 20 |
14.9 ± 1.84 20 |
19.5 ± 1.92 20 |
13.7 ± 1.52 20 |
|
Right coronary cusp |
17.5 ± 2.95 20 |
17.3 ± 2.28 20 |
19.4 ± 1.57 20 |
13.4 ± 1.75 20 |
|
Left coronary cusp |
17.3 ± 2.61 20 |
16.3 ± 2.00 20 |
19.1 ± 2.53 20 |
13.9 ± 1.30 20 |
Additionally, humans have much less muscular attachment surrounding the aortic valve
than animal hearts, an indication of their reduced cardiac output.20 Similarly, the human aortic valve at the annulus level possesses muscular attachment
along 43% of its circumference, compared to respective figures of 56%, 60%, and 57%
in porcine, bovine, and ovine valves.20,21 Additionally, a greater amount of myocardial tissue support is also present at the
aortic valve's right and left coronary cusp bases, distinguishing all three ovine,
bovine, and porcine valves from the human aortic valve. Notably, in clinical trials
involving sub-coronary transplantation, this increased muscle mass has resulted in
aortic-valvular stenosis.20
Differences in aortic valve leaflet shape and structure are also present, with only
porcine valve leaflet depths comparable to their human analogues, although specimen
analysis visualized more inter-species variation between individual leaflets in the
former.20 Variations in leaflet thickness are particularly important to make note of, as thin
and fragile leaflets, such as those observed in ovine species, may not be structurally
strong enough to support heavy pressure loads during clinical use for long periods.
Aortic Anatomy
Unlike the aspects of valvular anatomy, studies into the differences in the ascending
aorta between human and non-human species have not been extensively performed, highlighting
a current literature gap. However, morphometric studies have been documented to determine
the largest artery's structural characteristics in mammals. Primarily, compared to
the human heart, the porcine heart has only two head branches originating from the
aortic arch.
Dimensionally, the diameter of the proximal aorta among porcine species at its largest
part is about 21% lesser than that of their human analogues. Notably, unlike their
human counterparts, which exhibit a gradual diameter decrease in a tapering fashion,
the porcine aortic diameter decreases sharply from the descending thoracic aorta to
the abdominal aorta (Table 2). Conversely, while studies on the aortic anatomy of ovine species are inadequate,
the ascending aorta, while maintaining a similar aortic diameter to that of their
human counterparts after accounting for the changes in organ-to-body weight ratio,
is relatively short. Its implications shall be discussed in the next section.27 There is also a marked decrease in the number of elastic lamellae within ovine aorta,
greatly reducing its mobility as well.27
Table 2.
Dimensions of the Aorta.
| Measurement (mm) |
Human |
Porcine |
Bovine |
Ovine |
| Aortic annulus diameter |
23.0 ± 2.5 21 |
20.0 ± 1.2 21 |
48.0 ± 0.92 24 |
Not document-ed in adults |
| Thoracic aortic diameter at sinotubular junction |
27.2 ± 3.0 21 |
20.0 ± 0.9 21 |
Not documented in adults |
Not document-ed in adults |
| Abdominal aorta diameter (measured at level of superior mesenteric artery) |
22.0 ± 0.3 25 |
10.4 21 |
Not documented in adults |
Not document-ed in adults |
Legend: Standard deviations for abdominal aortic dimensions in pigs were not documented.
Finally, the bovine ascending aortic anatomy is the most reviewed of the three non-human
species described in this review article. The ‘bovine aortic arch' has been described
as the single most common congenital aortic anatomic variant within humans as well.
While this term itself is a misnomer, it is used to supposedly refer to the variant
within bovine species, which is characterized by a common single brachiocephalic trunk
trifurcating into bilateral subclavian vessels and a single bicarotid trunk, as opposed
to the more common human aortic arch, which splits into a single brachiocephalic trunk,
left common carotid, and left subclavian arteries.28,29
Similar to their ovine counterparts, little to no research has been done explicating
the dimensional differences in the aortic root diameter between bovines and humans,
elucidating the need for further research in this area.
Coronary Anatomy
The suitability of porcine species as an animal model in coronary arterial disease
is well established, with multiple morphological studies highlighting that porcine
coronary vasculature is similar to humans.33 In pigs, both coronary arteries arise from the aortic sinuses below the supravalvular
ridge, as is observed in human species, with one study highlighting that all tested
porcine models showed right coronary artery (RCA) dominance (humans typically exhibit
RCA dominance anywhere between 75 to 85%, depending on the chosen study analyzed).34 However, as with their human counterparts, certain inter-species variants are present
and should be considered in choosing a porcine animal model.34,35
With regards to the coronary arterial system, in contrast to their porcine and human
analogues, ovine species primarily have a left coronary type circulation; ergo, the
majority of the myocardium receives its blood supply through branches of the left
coronary artery.36 However, given that ovines do not possess an extensive coronary collateral network,
it may be still suitable to use their models for research. More specifically, although
there exists considerable literature that is descriptive of specific aspects of ovine
cardiac anatomy, little to no comparative research has been conducted to elucidate
the differences between ovine and human heart models, highlighting a significant literature
gap.36
The coronary vasculature of bovine species has also been studied and documented. In
all examined animals, the coronary ostia were located beneath the sinotubular junction,
as observed with their human counterparts.37 The dimensions of coronary ostia are listed in Table 3, but it is important to note that ovines are one of the most common veterinary species
to exhibit coronary artery anomalies, with examples of such abnormalities including
coronary-to-pulmonary artery fistulae and anomalous origin of the left coronary artery
from the pulmonary trunk. Consequently, their use as animal models to mimic the human
coronary system merits scrutiny before findings can be extrapolated.38,39
Table 3.
Dimensions of the Coronary Vasculature.
| Measurement (mm) |
Human |
Porcine |
Bovine |
Ovine |
| Left coronary ostia diameter |
4.8 ± 0.5 21 |
5 ± 0.5 21 |
7.1 ± 1.7 38 |
5.38 ± 1.59 39 |
| Right coronary ostia diameter |
3.7 ± 0.9 21 |
4.7 ± 0.5 21 |
5.3 ± 1.4 38 |
1.75 ± 0.44 39 |
| Coronary collateralization |
Limited |
Limited |
Anomalous |
Limited |
Suitability for use as Animal Clinical Models in Type-A Aortic Dissection Research
Having explored the anatomical differences between ovine, bovine, and porcine species,
the anatomic feasibility of using these as animal models to better our understanding
of Type-A AD treatment options shall now be explored.
Type-A ADs involve the ascending aorta, making this aspect of the model's anatomy
significantly important. Bovine aortic anatomy is particularly unhelpful for this
pathology, given the marked differences from humans, as elucidated previously.28 Indeed, the ‘bovine aortic arch effect' is an epidemiological term used to highlight
the linkage between ascending and thoracic aortic dilatation due to the aortic arch
anatomy within bovines, further exemplifying their unsuitability as animal models
in this context.40
Between the ovine and porcine species, each species seems to share some features with
that of humans while exhibiting some differences that affect their use as animal models.
For instance, while ovines maintain a uniform aortic diameter similar to that of humans,
their short immobile aorta could pose a challenge to graft repair within animal models.27 Conversely, despite of the larger aorta of pigs, the aortic diameter being nearly
a fifth lesser than that of humans could also affect the reproducibility of findings
to the latter. Consequently, it is difficult to assess which ovine or porcine models
is better for modeling Type-A ADs, at least from the ascending aortic anatomy perspective.
The aortic valvular anatomy is significant when choosing an appropriate animal model,
particularly with AD tears extending proximally into the aortic root.41 As indicated, variations in leaflet thickness are important, as the heavy pressure
loads exerted during clinical use can affect the structural stability of the animal
model. Consequently, species with relatively thinner valvular commissures, such as
in ovines, must be handled with due care. As a result, porcine models are preferred
to the other models.
Finally, the coronary vasculature of these animal models also has relevance to the
pathology of Type-A ADs, especially with tears arising in the aortic root, or even
with any more distal tears causing dissections in the proximal sinotubular junction,
both of which would affect the coronary supplies, and thus consequently cause ischemia
of the cardiac musculature. Given that bovine species exhibit the most coronary artery
anomalies, their use as an animal model in better understanding the various treatment
options for Type-A ADs is hence not justified, given that these findings would not
necessarily accurately represent what we might observe in humans.38,39
Between porcine and ovine species, the coronary vasculature is similar to that of
humans. However, as indicated, much more research has been conducted on the coronary
arterial supply of pigs, with little to no comparative research being conducted on
their ovine counterparts, and as such, the former takes current precedence when selecting
an animal model for Type-A AD research.
Limitations of this Review & Insights on Future Research
Comparing ovine, porcine, and bovine cardiac anatomy and their use as animal models
will undoubtedly provide important new insights into new endovascular treatment options
for Type-A AD. However, as explored in this review, several limitations exist, with
a prominent example being the lack of literature on anatomical differences among these
species. First, there is a lack of information on the microscopic anatomical differences
in cardiac anatomy among species, such as the anatomical variances in the layers of
the aorta among porcine, ovine, and bovine species. Additionally, although considerable
literature describes either very general or very specific aspects of mammalian cardiac
anatomy, little quantitative, truly comparative research has been conducted. These
tie into our final limitation, which is the nature of this review itself. As a narrative
review, while it provides information about the current state of research and addresses
future directions and possible clinical applications, it was limited in comprehensive
results analysis. Potentially, a systematic review might yield more comprehensive
data and identify any biases or random errors. In the long term, the authors encourage
researchers currently using animal models of cardiovascular disease to publish their
findings and add to the literature to allow such translation to human interventions.
Conclusion
The introduction of intravascular stent-grafts as a surgical treatment option for
Type-A ADs represents one of the most successful innovations in cardiothoracic surgery
within the last few decades. However, lingering high numbers of patient mortality
rates despite surgical intervention highlights the accelerated need for our better
understanding of novel treatment options for this disease, explicating the necessity
of developing an appropriate animal clinical model. From a strictly anatomical standpoint,
bovine species do not meet this need, given the significant variations in aortic arch
anatomy, the lack of literature on aortic valvular anatomy, and finally, the significant
variation in coronary artery anatomy. However, both porcine and ovine species appear
to be suitable options as animal models for proximal aortic endovascular treatment,
with the former possessing a slight advantage, given similarities in the coronary
artery and aortic valve anatomy to their human analogues. The identification of appropriate
animal models will provide knowledge for further insight into the available endovascular
treatment options for Type-A ADs and consequently needs to be hastened.
Summary - Accelerating Translation
Open heart surgery has seen a marked evolution over the last century, with improving
technologies and advancing surgical techniques providing better outcomes to patients
worldwide. In particular, the advent of minimally-invasive surgical repair of one's
blood vessels, also known as endovascular repair, has heralded a paradigm shift in
this field, providing patients with quicker recovery times and offering life-saving
surgery to a significantly larger proportion of people who would otherwise be too
frail for such a delicate procedure. The usage of endovascular repair has greatly
increased for diseases involving the descending aorta, but has currently been used
with limited scope for the ascending aorta, given the latter's proximity to the heart.
Consequently, appropriate animal models are urgently needed to improve our understanding
of endovascular treatment of ascending aortic dissections, also known as Stanford
Type-A ADs, a condition with a mortality rate of nearly 100% if left untreated for
longer than a fortnight.
This narrative review aims to provide a current literature summary on the subject,
including the gross anatomical differences among adult porcine, ovine, and bovine
species, compared with those of their human counterparts, as well as specific valvular
and coronary vasculature measurement variances. An electronic search of Cochrane Library,
PubMed, and Ovid Medline databases from January 1965 to June 2020 was performed, with
the search limited to articles published in English. In total, twenty-three research
papers were included and synthesized for this review.
Several conclusions were drawn, with our findings revealing that while macroscopic
anatomy remains grossly similar among these species, differences in valvular leaflet
shape are present, with porcine and ovine models possessing anatomic characteristics
that are comparable to their human counterparts. Inter-species differences, concerning
the anatomy of the ascending aorta, remain an area of ongoing research, and have not
been extensively studied at present, highlighting a literature gap. Conversely, multiple
studies have highlighted that porcine coronary vasculature, or the arteries which
supply the heart muscle itself, is similar to that of humans.
In summary, both porcine and ovine species are suitable as appropriate animal models
for examining the feasibility of endovascular stent-grafts for ascending ADs. However,
given the similarities in coronary and aortic valve anatomy with humans, porcine models
are better suited for this purpose.
Conflict of Interest Statement & Funding
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Author Contributions
Project Administration, Supervision, Writing-Review & Editing: AS, MY, JS; Conceptualization:
AS, MY; Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing-Original
Draft Preparation: AS.
Acknowledgments
None.
References
1. Cesarovic N, Lipiski M, Falk V, Emmert M. Animals in cardiovascular research: Clinical relevance and translational limitations
of animal models in cardiovascular medicine. EHJ. 2020;41(2):200–3.
2. Criado F. Aortic Dissection: A 250-Year Perspective. Tex Heart Inst J. 2011; 8(6):694–700.
3. Fujimura N, Kawaguchi S, Obara H, Yoshitake A, Inoue M, Otsubo S et al. Anatomic Feasibility of Next-Generation Stent Grafts for the Management of Type A
Aortic Dissection in Japanese Patients. Circ J. 2017;81:1388–94.
4. Chiu P, Miller DC. Evolution of surgical therapy for Stanford acute type A aortic dissection. Ann Cardiothorac Surg. 2016;5(4):275–95.
5. Scholl F, Coady M, Davies R. Interval or Permanent Nonoperative Management of Acute Type A Aortic Dissection. JAMA Surgery. 1999; 134(4):402–6.
6. Auer J, Berent R, Eber B. Aortic Dissection: Incidence, Natural History and Impact of Surgery. Journal of Clinical and Basic Cardiology. 2000;3(3),151–4.
7. Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation 1995;92(2):113.
8. Becker H, Jauch K. Vascular Surgery. 1st Edition. Berlin: Springer-Verlag; 1989. p. 349-60
9. Shah A, Khoynezhad A. Thoracic endovascular repair for acute type A aortic dissection: operative technique. Ann Cardiothorac Surg. 2016;5(4):389–96.
10. Kreibich M, Rylski B, Kondov S, Morlock J, Scheumann J, Kari F et al. Endovascular treatment of acute Type A aortic dissection—the Endo Bentall approach. J Vis Surg. 2018;1(4):69.
11. Heilmann C, Stahl R, Schneider C, Sukhodolya T, Siepe M, Olschewski M et al. Wound complications after median sternotomy: a single-centre study. Interact Cardiovasc Thorac Surg. 2013;16(5):643–8.
12. Luciani G, Lucchese G. Minimal-access median sternotomy for aortic valve replacement. J Thorac Dis. 2013;5(Suppl 6):S650–3.
13. Sarkar M, Prabhu V. Basics of cardiopulmonary bypass. Indian J Anaesth. 2017;61(9):760–7.
14. Zanotti G, Reece TB, Aftab M. Aortic Arch Pathology: Surgical Options for the Aortic Arch Replacement. Cardiol Clin. 2017;35(3):367–85.
15. Nordon IM, Hinchliffe RJ, Morgan R, Loftus IM, Jahangiri M, Thompson MM. Progress in endovascular management of type A dissection. Eur J Vasc Endovasc Surg. 2012;44(4):406–10.
16. Kreibich M, Soekeland T, Beyersdorf F, Bavaria J, Schröfel H, Czerny M et al. Anatomic feasibility of an endovascular valve–carrying conduit for the treatment of
type A aortic dissection. J Thorac Cardiovasc Surg. 2019;157(1):26–34.e1.
17. Harky A, Al-Adhami A. Stenting in type A aortic dissection: fantasy or reality? J Vis Surg. 2018;4(161):1–3.
18. Mangialardi N, Serrao E, Ronchey S, Kasemi H, Orico M. Endovascular Treatment of Type A Dissections. Endovascular Today. 2013 Nov. Available from: https://evtoday.com/articles/2013-nov/endovascular-treatment-of-type-a-dissections
19. University of Minnesota. Comparative Anatomy of the Valves. Available from: http://www.vhlab.umn.edu/atlas/comparative-anatomy-tutorial/external-anatomy.shtml. Last updated [Jan 14, 2019]; cited [Jan 20,2020].
20. Sands M, Rittenhouse E, Mohri H, Merendino K. An Anatomical Comparison of Human, Pig, Calf, and Sheep Aortic Valves. Ann Thorac Surg. 1969;8(5):407–14.
21. University of Minnesota. Comparative Anatomy of the Valves. Available from: http://www.vhlab.umn.edu/atlas/comparative-anatomy-tutorial/valves.shtml. Last updated Jan 14,2019; cited Jan 20,2020.
22. Wang C, Lachat M, Regar E, von Segesser L, Maisano F, Ferrari E. Suitability of the porcine aortic model for transcatheter aortic root repair. Interact Cardiovasc Thorac Surg. 2017;26(6):1002–8.
23. Tao L, Xianhao B, Yuxi Z, Ziwen L, Ziyi X, Zhaoxiang Z et al. Thoracic aortic computed tomography angiography in porcine: establishment of a baseline
for endovascular evaluation of the ascending aorta. Interact Cardiovasc Thorac Surg. 2020:31(2):248–53
24. Khan S, Islam M. Studies on the Prospect of Bioprostheses by Bovine Aortic Valve for Human Use. Bangladesh Med Res Counc Bull. 1991;17(2):75–80
25. Hyun Joh J, Ahn H, Park H. Reference Diameters of the Abdominal Aorta and Iliac Arteries in the Korean Population. Yonsei Med J. 2013;54(1):48–54.
26. Jonker F, Mojibian H, Schlösser F, Botta D, Indes J, Moll F et al. The Impact of Hypovolaemic Shock on the Aortic Diameter in a Porcine Model. Eur J Vasc Endovasc Surg. 2010;40(1):564–71.
27. DiVincenti L, Westcott R, Lee C. Sheep (Ovis aries) as a Model for Cardiovascular Surgery and Management before, during,
and after Cardiopulmonary Bypass J Am Assoc Lab Anim Sci. 2014;53(5):439–48.
28. Dumfarth J, Chou A, Ziganshin B, Bhandari R, Peterss S, Tranquilli M et al. Atypical aortic arch branching variants: A novel marker for thoracic aortic disease. J Thorac Cardiovasc Surg. 2015;149(6):1586–92.
29. Layton K, Kallmes D, Cloft H, Lindell E, Cox V. Bovine Aortic Arch Variant in Humans: Clarification of a Common Misnomer. AJNR Am J Neuroradiol. 2006;27(7):1541–2.
30. Torad F, Amer M, Shamaa A, Elsherpieny E. Echocardiographic measurements and indices in normal adult buffalo (Bubalus bubalis). Journal of Applied Animal Research. 2016;45(1):336–41.
31. Devereux R, Simone G, Arnett D, Best L, Boerwinkle E, Howard B et al. Normal Limits in Relation to Age, Body Size and Gender of Two-Dimensional Echocardiographic
Aortic Root Dimensions in Persons ≥15 Years of Age. Am J Cardiol. 2012;110(8):1189–94.
32. Braun U, Schweizer T. Determination of Heart Dimensions in Cattle via 2-D-mode Echocardiography. Berl Munch Tierarztl Wochenschr. 2001;114(2):46–50.
33. Sahni D, Kaur G, Jit H, Jit I. Anatomy & Distribution of Coronary Arteries in Pig in Comparison With Man. Indian J Med Res. 2008;127(6):564–70/
34. Weaver M, Pantely G, Bristow J, Ladley H. A Quantitative Study of the Anatomy and Distribution of Coronary Arteries in Porcine
in Comparison With Other Animals and Man. Cardiovasc Res. 1986;20(12):907–17.
35. Gómez F, Ballesteros L. Evaluation of coronary dominance in pigs; a comparative study with findings in human
hearts. Arq. Bras. Med. Vet. Zootec. 2015;67(3):783–9.
36. Frink R, Merrick B. The Sheep Heart: Coronary and Conduction System Anatomy With Special Reference to
the Presence of an Os Cordis. Anat Rec. 1974;179(2):189–200.
37. Scansen B. Coronary Artery Anomalies in Animals. Vet. Sci. 2017;4(2):20.
38. Barszcz K, Polguj M, Klećkowska-Nawrot J, Goździewska-Harłajczuk K, Olbrych K, Czopowicz M. Morphometry and topography of the coronary ostia in the European bison. Folia Morphol. 2019;79(1):105–12.
39. Gómez F, Cortés L, Ballesteros L. Morphological characterisation of the coronary arteries in African sheep (Ovis orientalis).
Differential analysis with those of humans and other animal species. Folia Morphol. 2018;78(1):63–70.
40. Pham T, Martin C, Elefteriades J, Sun W. Biomechanical characterisation of ascending aortic aneurysm with concomitant bicuspid
aortic valve and bovine aortic arch. Acta Biomater. 2013;9(8):7927–36.
41. Ho S. Structure and anatomy of the aortic root. Eur J Echocardiogr. 2009;10(1):3–10
Abhishekh Srinivas, 1 BMedSc/MD. Department of Surgery, Monash University, School of Clinical Sciences
at Monash Health, Monash Medical Centre, Melbourne, Australia.
Ming Yii, 2 MBBS, FRACS (Vascular), MPH. Department of Surgery, Monash University, School of
Clinical Sciences at Monash Health, Monash Medical Centre, Melbourne, Australia.
Julian A. Smith, 3 MBBS, MS, FRACS (Cardiothoracics), FAICD. Department of Surgery, Monash University,
School of Clinical Sciences at Monash Health, Monash Medical Centre, Melbourne, Australia.
About the Author: Abhishekh is a junior medical doctor currently working at The Alfred in Victoria,
Australia. He is a recent graduate from Monash University, where he also completed
a Bachelor of Medical Science (Honors) in ascending aortic dissections from the School
of Clinical Sciences at Monash Health.
Correspondence: Abhishekh Srinivas, Address: Dept. of Surgery, Monash Medical Centre Level 5, Block
E 246, Victoria, Australia. Email: abhishekhsrinivas@gmail.com
Editor: Francisco J. Bonilla-Escobar
Student Editors: Madeleine J. Cox & Nikoleta Tellios
Copyeditor: Johnmark Boachie
Proofreader: Lourdes A. Medina-Gaona
Layout Editor: Lucianne A. Odiero
Process: Peer-reviewed
Cite as: Srinivas A, Yii M, Smith J. Comparative Review of Large Animal Models for Suitability
of Proximal Aortic Endovascular Repair. Int J Med Stud. 2022 Apr-Jun;10(2):185-91.
Copyright © 2022 Abhishekh Srinivas, Mong Yii, Julian A. Smith
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 2, March 2022

