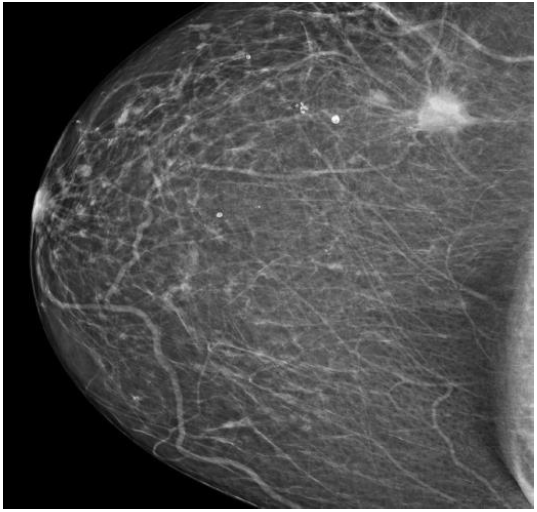
Screening Mammogram Showing Spiculated Mass in Right Breast.
Highlights:
doi: http://dx.doi.org/10.5195/ijms.2020.633
Volume 8, Number 3: 295-298
Received 25 06 2020: Rev-request 14 07 2020: Rev-recd 31 08 2020: Accepted 11 11 2020
ABSTRACT
Background:Lymphatic spread of breast cancer is currently well understood and can be assessed in breast cancer patients through the use of lymphatic mapping with sentinel node biopsy, computed tomography or positron emission tomography. However, this case highlights a unique pattern of lymphatic spread of unknown etiology.
The Case:A 73-year-old female with two distinct primary carcinomas, right-sided invasive lobular carcinoma of the breast and left-sided adenocarcinoma of the lung. We discuss the predictable and unpredictable lymphatic drainage of the lobular carcinoma, including ipsilateral drainage to the axillary chain and suspected contralateral hilar and mediastinal lymph node metastasis.
Conclusion:The unique lymphatic spread of the breast cancer in this case emphasizes the use of lymphatic mapping for staging of disease and staining biopsied tissue samples for tumor markers to guide treatment. Additional anatomic research in this patient or supporting reported cases are needed to determine the frequency and cause of aberrant lymphatic drainage of primary invasive lobular carcinoma of the breast.
Keywords: Lobular carcinoma; Breast cancer; Adenocarcinoma of lung (Source: MeSH-NLM).
Invasive lobular carcinoma (ILC) comprises approximately 10% of all breast cancers.1 The malignant cells line up in a single file in the stroma and usually do not form a distinct, palpable mass. Overall, the prognosis tends to be good, due to the low grade of the tumor and its nature of being estrogen receptor positive (ER +).2 However, ILC tends to be multifocal and multicentric and can involve both breasts.3 Such characteristics have led to cases of distant metastases involving the peritoneum, ovaries, and uterus.4 The vast majority of breast malignancies tend to spread via axillary lymph nodes, but there can also be nodal metastases outside of the axillary lymph nodes, involving the internal mammary, infraclavicular, and supraclavicular lymph nodes.5 Furthermore, breast malignancies, using lymphatic and hematogenous routes, can have pulmonary involvement, called pulmonary lymphangitic carcinomatosis.6
Adenocarcinoma of the lung is the most common type of lung cancer and can quickly spread to distant sites via lymphatic and hematogenous routes, which often results in stage IV disease by the time of patient presentation.7
Here, we present an interesting case of a primary, right-sided invasive lobular carcinoma with ipsilateral axillary nodal involvement and primary, left-sided adenocarcinoma of the lung with suspected ipsilateral hilar and mediastinal lymph node metastasis, which actually turned out to be invasive lobular carcinoma. Informed consent of publication was obtained from the patient prior to submission/publication.
The patient is a 73-year-old African American female with no family history of breast or ovarian cancer who initially presented due to a right breast mass palpated upon routine self-breast examination. She had a screening mammogram completed, which showed a 2.5cm spiculated mass in the right breast at the 6 o'clock position, 16cm from the nipple along with a 1.1cm asymmetry anterior to the mass and a 0.8cm asymmetry in the right retroareolar region (Figure 1). The radiologist assessment was BI-RADS 0 (Breast Imaging Reporting and Database System), and additional imaging was recommended.
Figure 1Screening Mammogram Showing Spiculated Mass in Right Breast.

Two weeks later, the following diagnostic mammogram showed a 2.3cm irregular, spiculated mass in the right breast at the 9 o'clock position, 17cm from the nipple along with a 0.7cm mass, 2cm from the nipple. Whole breast ultrasound showed a solid mass in the right 8 to 9 o'clock position, highly suspicious for malignancy along with an abnormal lymph node in the right axilla. The radiologist assessment this time was BI-RADS 5: highly suggestive of malignancy, so biopsies of the lesion and lymph node were recommended.
The patient was seen by a breast surgeon, who examined the patient and noted the patient's breasts to be enormous and very pendulous with palpation of some nodularity in the extreme outer right breast, but no lymph nodes were palpated in the axillary and supraclavicular regions.
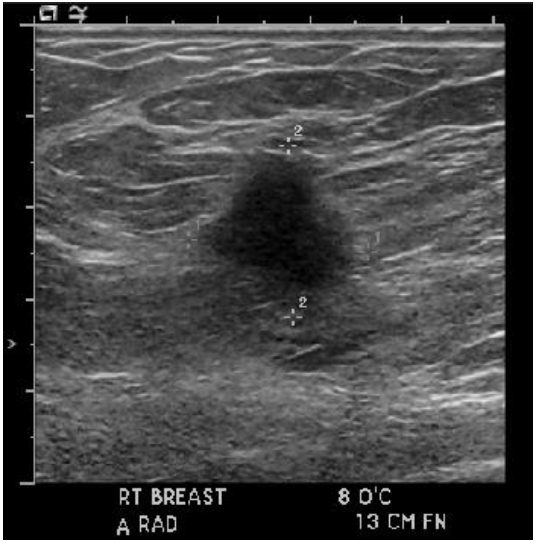
The following day, patient underwent an ultrasound-guided core biopsy with multiple cores taken from the primary lesion in the right breast at the 8 o'clock position, 13cm from the nipple (Figure 2). The 5-6mm satellite lesion was seen 3-4mm away from the primary lesion, but no biopsy samples were taken. Multiple cores were taken from an enlarged right axillary lymph node, which showed cortical thickening with compromise of the hilum that measured 1cm. The pathology report that followed showed invasive lobular carcinoma with positive axillary metastasis.
Figure 2Ultrasound Image Showing Right Breast Mass Prior to Biopsy.
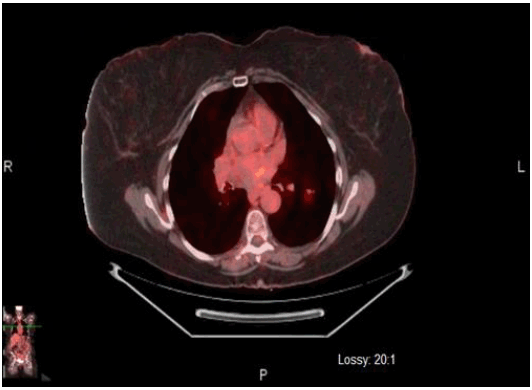
Breast-specific gamma imaging (BSGI) and Positron emission tomography-computed tomography (PET-CT) were ordered one week later. The BSGI showed a 2cm diameter area of intense focal uptake in the right breast at 9 o'clock, 15cm from the nipple with no evidence of multifocal, multicentric disease. The PET-CT findings included: an irregular right lateral breast mass, consistent with known right breast carcinoma, mildly enlarged right axillary lymph nodes consistent with nodal metastases (Figure 3), and an irregular nodule within the superior segment of the left lower lobe, which was concerning for metastasis or concurrent primary bronchogenic carcinoma (Figure 4).
Figure 3PET Image Showing Right Lateral Breast Mass with Fluorodeoxyglucose Uptake.

PET Image Showing Fluorodeoxyglucose Uptake in Superior Segment of Left Lower Lobe.
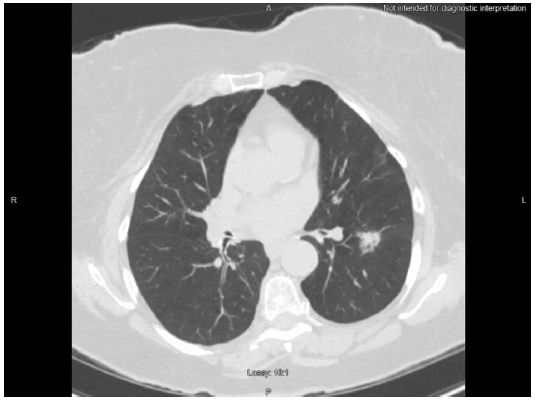
Based on such findings, the breast surgeon and interventional radiologist agreed upon a CT-guided biopsy of the left pulmonary lesion (Figure 5) 2 weeks after the imaging studies were completed. Two 20-gauge core biopsy specimens were obtained with no complications, and the pathologist reported well differentiated pulmonary adenocarcinoma with predominant lepidic growth pattern. The specimens stained positively for cytokeratin 7, napsin-A, and TTF-1 (thyroid transcription factor-1). The major risk factor for the patient was a history of tobacco use, which started at 17 years of age.
Figure 5CT showing left lung lesion prior to biopsy
At this point, the patient's diagnoses were stage II carcinoma of the breast and a primary tumor of the left lung. Three weeks later, the patient subsequently underwent a right needle-localized lumpectomy and axillary dissection. The pathology report confirmed invasive lobular carcinoma, and 2/2 lymph nodes tested positive for metastatic lobular carcinoma. The tumor was ER (estrogen receptor) positive, PR (progesterone receptor) negative, and HER2 (human epidermal growth factor receptor 2) negative. The staging of the breast cancer was T1c N1 M0 stage IIa invasive lobular carcinoma.
The patient was referred to an oncologist and cardiothoracic surgeon. One month later, she had a left lower lobectomy and mediastinal lymph node dissection performed. An 18.5 × 12.0 × 4.0cm lobe of the left lung that weighed 173.4g was removed. The pathology report showed moderately differentiated adenocarcinoma with lepidic predominance; all the vascular and bronchial margins were free of tumor. However, the pathology report showed the left mediastinal lymph nodes to actually be invasive lobular carcinoma: 1 of 1 peribronchial, 5 of 6 interbronchial, 1 of 1 subcarinal, 1 of 1 aortopulmonary window, 1 of 1 left inferior pulmonary ligament, and 1 of 1 hilar. The hilar lymph nodes stained positive for pankeratin and GATA3 (transcription factor and breast cancer marker) (Takaku) and negative for TTF-1 (Schilsky) and CD68. They were strongly positive for ER 100%, positive for PR 2%, and HER2 negative. Based on such histological characteristics, the left hilar lymph nodes were most consistent with metastatic invasive lobular carcinoma and not lung adenocarcinoma. The patient's diagnoses were changed to stage IV carcinoma of the breast and stage IA2 (T1b, N0, M0) carcinoma of the lung. She is currently undergoing chemotherapy with letrozole and Ibrance (palbociclib).
Here we presented a unique case of primary lobular carcinoma of the right breast, primary invasive adenocarcinoma of the left lower lung lobe with pankeratin positive, GATA3 positive, ER positive left peribronchial lymph node involvement. Lymphatic spread of breast cancer is thought to be well understood. This case highlights a unique pattern of lymphatic spread of unknown etiology. Review of other case reports indicates the pattern of lymph node involvement in this patient with invasive lobular carcinoma of the breast is quite unique. While other cases were found on spread of invasive lobular carcinoma to the contralateral breast and aberrant lymphatic drainage patterns in recurrent breast cancer after treatment, no additional cases were found in the literature of spread of invasive lobular carcinoma to contralateral hilar and mediastinal lymph nodes in a patient with no previous history or treatment of breast cancer.
Lymphatic drainage of the breast drains ipsilaterally through the axillary, transpectoral and internal mammary pathways.8 Further drainage occurs into “lymphatics that course along the axillary and contiguous subclavian vein. From here, the lymphatics may drain directly into the jugulosubclavian confluence or initially pass through the jugular and bronchomediastinal lymphatics.”8 The variance of lymphatic drainage seen in various tumors may be attributed to the quality of the lesions, whether it is palpable or nonpalpable, and the location of the lesion within the breast, either in the right outer, right inner, left outer, or left inner quadrant or in the center. According to Estourgie et al, both palpable and nonpalpable lesions can drain toward the internal mammary chain, but this pattern is more commonly seen with nonpalpable lesions.9 Furthermore, Estourgie et al. reveal 97.1% of palpable lesions in the left outer quadrant should be expected to drain to the axillary lymphatic bed while 26.1% of palpable lesions in the same location drained to the internal mammary chain of lymphatics.9 Because of the location of the primary breast tumor in the left outer quadrant (9 o'clock position of the right breast), the most predictable pattern of lymphatic drainage in this patient would be to the axillary nodes. While this patient had involved lymph nodes in the axillary chain, including sentinel nodes, stain positive for the invasive lobular carcinoma, additional lymphatic drainage to the contralateral hilar and mediastinal lymph nodes without ipsilateral hilar and mediastinal lymph node involvement was unique and not currently explained by the literature.
The question raised by the physicians caring for this patient was, how did the breast cancer spread to a single, contralateral region of lymph nodes without involvement of other ipsilateral or contralateral lymph node beds? Could this be an anatomic variant in this patient? Were there additional factors that allowed the spread of the tumor to a contralateral lymphatic bed, or was the cancer undiagnosed for a length of time adequate enough for the lobular carcinoma to contralaterally spread? According to Sharma et al.,8 obstruction of normal lymphatic flow allows for development of collateral lymphatic drainage pathways, including internal mammary and mediastinal lymphatics. Lymphatic mapping with sentinel node identification in this patient identified drainage of the primary tumor to the axillary nodes. An anatomical variant or development of collateral pathways not assessed during the lymphatic mapping could explain the unique spread of the invasive lobular carcinoma in this patient. This case may support the expansion of lymphatic mapping into further lymphatic regions to identify spread of the tumor and may prompt further developments and advancements in lymphatic mapping as it pertains to breast cancer. The unpredictable lymphatic spread in this case prompts the discussion of the appropriateness of routine PET scans prior to treatment in breast cancer patients with no evidence of additional lymphatic spread on CT. CT is commonly used in cancer imaging to determine lymph node involvement. Because involved lymph nodes appear normal on CT, fluorine-18 fluorodeoxyglucose (FDG) PET can be used for more accurate detection of lymphatic spread of the primary tumor and relevant lymph node involvement.8 Additional reports and studies are needed to determine the incidence of contralateral spread of lobular carcinoma of the breast to unusual lymphatic beds to determine the benefit of FDG-PET in conjunction with current CT scanning on patients with suspected additional extra-axial or contralateral lymph node involvement.
During the initial assessment of the patient, the involved hilar and mediastinal lymph node was suspected to be secondary to the primary adenocarcinoma of the left lung by the treatment team. Only through further staining and discussion was the origin of the carcinoma in the hilar and mediastinal lymph nodes discovered.
The complexity of this case illustrates the importance of staining biopsied tissue samples for tumor markers to accurately stage the disease and to ensure a chemotherapy regimen and further treatment is appropriate. Further research is needed to determine the impact of the aberrant lymphatic drainage of primary invasive lobular carcinoma as seen in this patient.
Special thanks to Charles Scarborough, M.D., Ms. Vanessia Rodriguez, and St. Francis Emory Healthcare for their contributions to this case report.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: RWL, ED. Data Curation: RWL. Formal Analysis, Investigation: ED. Visualization: RWL. Writing – Original Draft Preparation, Writing – Review & Editing: RWL, ED.
1. Cocquyt V, Van Belle S. Lobular carcinoma in situ and invasive lobular cancer of the breast. Curr Opin Obstet Gyn. 2005 Feb;17(1):55-60.
2. McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and 'omics. Breast Cancer Res. 2015 Jan;17(1):12.
3. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004 Feb;6(3):R149-R156.
4. Osaku T, Ogata H, Magoshi S, et al. Metastatic nonpalpable invasive lobular breast carcinoma presenting as rectal stenosis: a case report. J Med Case Rep. 2015 Mar;9:88.
5. Aukema TS, Straver ME, Vrancken Peeters MJTFD, Russell NS, Gilhuijs KGA, Vogel WV, et al. Detection of extra-axillary lymph node involvement with FDG PET/CT in patients with stage II-III breast cancer. European Journal of Cancer. 2010 Dec;46(18):3205-3210.
6. Burns Z, Omar L, Compton L, Hayes J, Sahoo S, Merchant K. Lymphangitic spread of invasive lobular carcinoma to the contralateral breast. Clin Imaging. 2019 Nov;58:187-190.
7. Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016 Mar;35(1):75-91.
8. Sharma A, Fidias P, Hayman LA, Loomis SL, Taber KH, Aquino SL. Patterns of Lymphadenopathy in Thoracic Malignancies. RadioGraphics. 2004 Mar-Apr;24(2):419-434.
9. Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg. 2004 Feb;239(2), 232-237.
Rina Won Lee, 1 BS, Mercer University School of Medicine, Macon, Georgia, United States
Emily Denney, 1 BS, Mercer University School of Medicine, Macon, Georgia, United States
About the Author: Rina Lee is a current third year medical student of Mercer University School of Medicine, Columbus, Georgia, USA of a four-year program. She is a member of the Alpha Omega Alpha Honor Medical Society. Emily Denney is also a current third year medical student at Mercer University School of Medicine, Columbus, Georgia, USA of a four-year program. She is a member of the Gold Humanism Honor Society.
Correspondence: Rina Won Lee. Address: 1550 College St, Macon, GA 31207, United States. Email: rinalee25@gmail.com
Editor: Paul MacDaragh Ryan Student Editors: Samreen Fathima & NIkoleta Tellios Copyeditor: Adnan Mujanovic Proofreader: Sohaib Haseeb Layout Editor: Annora A. Kumar
Cite as: Lee RW, Denney E. Aberrant Lymphatic Drainage of Primary Invasive Lobular Carcinoma with Concurrent Primary Lung Adenocarcinoma: A Case Report. Int J Med Students. 2020 Sep-Dec;8(3):295-8.
Copyright © 2020 Rina Won Lee, Emily Denney
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 8, NUMBER 3, December 2020