Flowchart of the Study.
José Adrián Yamamoto-Moreno1, Cecilia Pineda-Aguilar1, Samuel Ruiz-Pérez2, Gloria Liliana Gortarez-Quintana2, Marco Antonio Ruiz-Dorado3
doi: http://dx.doi.org/10.5195/ijms.2020.625
Volume 8, Number 3: 220-230
Received 23 06 2020: Rev-request 04 07 2020: Rev-request 19 08 2020: Rev-recd 05 07 2020: Rev-recd 21 08 2020: Accepted 24 11 2020
ABSTRACT
Background:Healthcare workers (HCW) are a high-risk group for contraction of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The aim of this study was to estimate the effect size of being a HCW and acquiring coronavirus disease 2019 (COVID-19) at the Mexican Institute of Social Security (IMSS) in Tijuana, Mexico.
Methods:A cross-sectional study of the Epidemiologic Surveillance Online Notification System database was conducted, including entries from Tijuana City between March 11, 2020 to May 1, 2020. Multiple imputation was performed for SARS-CoV-2 RT-PCR result where data was missing. Prevalence odds ratios (POR) were calculated to estimate the effect size of HCWs contracting COVID-19 compared to the general population (GP).
Results:From a total of 10,216 entries, 6,256 patients were included for analysis. HCW status was significantly associated with higher odds of acquiring COVID-19, (POR=1.730, 95% Confidence Interval [95%CI]=1.459;2.050). Nurses had double odds (POR=2.339, 95%CI=1.804;3.032) than the GP. Physicians had a POR=1.828 (95%CI=0.766;1.380). Resident physician status was double the likelihood of the GP (POR=2.166, 95%CI=0.933;5.025). Meanwhile, being an intern had a protective factor (POR=0.253, 95%CI=0.085;0.758). Among medical specialties, emergency medicine had the highest exposure-effect association, followed by anesthesiologists.
Conclusion:HCW had up to 73% increased odds of acquiring COVID-19 than the GP in Tijuana, Mexico. Nurses were the group with the highest likelihood out of all HCW, as a result of prolonged and close contact with patients. Emergency medicine and anesthesiology were the medical specialties with the highest odds of infection because they frequently perform aerosol-generating procedures.
Keywords: COVID-19; Coronavirus; SARS-CoV-2; Health personnel; Healthcare workers (Source: MeSH-NLM).
Healthcare workers (HCW) are a high-risk population for acquiring COVID-19. 1-2 Viral transmission has multiple pathways, the most studied being through respiratory droplets, with increased estimates of transmission of SARS-CoV-2 compared to influenza.3,4 For HCW, the workplaces at greater risk of infection are the respiratory and infectious disease departments, the ICU, and the operating room, given the prolonged times exposed to patients and the performance of aerosol-generating procedures.5,6 On January 2020, Category A specifications for control and prevention of infection measures were recommended by Chinese Centers for Disease Control and Prevention as their country was the first to experience the pandemic, even though COVID-19 was considered a Group B infectious disease by the World Health Organization.7,8 These measures focus on preventing transmission primarily through respiratory droplets during the execution of high-risk procedures such as endotracheal intubation, extubation, non-invasive ventilation, CPR, bronchoscopy, surgery, and autopsies.9
However, many asymptomatic and mild cases, which are still infectious, continue to seek medical attention for other health problems at primary care clinics and emergency departments, contributing to the increase in the number of cases.4,10 Taking this into consideration, primary care and emergency physicians are considered to be most at risk for acquiring SARS-CoV-2 infection, from subclinical to some symptomatic cases.11,12 Furthermore, different modes of viral transmission are still being researched, with new recommendations on the management and handling of fecal matter13 and corpses of confirmed COVID-19 cases.14 Although vertical transmission has not been demonstrated, there has been reports of pregnant women admitted with suspected COVID-19 at the end of gestation giving birth to newborns with positive SARS-CoV-2 test results.15
As a result of the uncertainty regarding disease transmission, severity, and mortality, access to some resources, such as face masks, sanitizers, and thermometers were soon scarce. At present, actions are being enforced to minimize the risks in the workplace with measures such as filtering at entry points, sanitizing hospitals, and continually providing personal protecting equipment (PPE) to the medical staff. Despite this, many HCWs in Mexico still feel vulnerable and question whether the PPE with which they are provided is sufficient.9 In other countries, HCW screening has been proposed, as they are considered amplifiers of nosocomial and community transmission.6
Regardless, the measures implemented have not been sufficient to contain the escalating number of cases. COVID-19 outbreaks have been reported all across Mexico, and several hospitals have notified of outbreaks internal to the hospital involving HCWs.9 The increase in the number of cases among the general population (GP) has also been reflected in HCWs,2,16,17 with sustained rises of confirmed cases. On April 24, 2020, 1,934 HCWs had a positive RT-PCR result for SARS-CoV-2, which represented 15% of the total (12,872) confirmed cases up to that day. The affected HCWs were distributed as follows: 47% physicians, 35% nurses, 15% other HCWs, 1% dentists and 1% laboratory staff, with as many as 4,148 HCWs temporarily removed from the workforce due to infection.18
Thus, the aim of this study was to estimate the effect size of being a HCW and acquiring COVID-19 at the Mexican Institute of Social Security (IMSS) in Tijuana, a US-border city in Mexico. As secondary analyses, risk estimates were stratified by HCW categories, by physician hierarchies, and by medical specialties.
A cross-sectional database study was conducted using data from the IMSS's Epidemiologic Surveillance Online Notification System (SINOLAVE), an internal network database that includes the records of COVID-19 suspected cases reported from different IMSS centers in Mexico. As this was secondary research from an institutional database, it was exempt from IRB review at IMSS.
The data for the study was extracted on May 11, 2020 and it corresponded to the entries recorded from March 11, 2020 to May 1, 2020. The data extraction criteria from SINOLAVE database were subset records from the Baja California delegation, including healthcare units from “all regimes”. Additional information about specific occupations of patients identified as HCWs was manually obtained through social security number (SSN) from electronic medical records before concealing subject identities for further analysis.
The SINOLAVE database consists of the following items: patient SSN, registry date, symptoms onset date, occupation and employer, clinical history including presence or absence of signs and symptoms, personal medical history (including chronic disease, tobacco smoking, alcohol consumption and pregnancy status, as well as history of travel and contact with COVID-19 cases and/or animals), results from RT-PCR for SARS-CoV-2 from nasopharyngeal or oropharyngeal swabs or specimens from lower respiratory tract secretions, treatment, and outcomes from primary and secondary healthcare systems.
The database was filtered to only include patients of all ages registered in Tijuana, Mexico, which corresponded to those notified from primary care centers number 7, 18, 19, 27, 33, 34, 35 and 36, and secondary care centers number 1 and 20. Individuals without complete personal and clinical history were excluded and duplicated or triplicated entries were eliminated. The first chronological record or the one that fulfilled severe acute respiratory infection (SARI) criteria was kept if records were registered twice at the same healthcare level. If duplicates were reported by different healthcare levels, the entry kept was from the highest healthcare level that included a reported laboratory test result. Data was recorded in a way that the identity of the human subjects could not be ascertained.
Patients whose registered occupation was “physician”, “nurse”, “laboratory staff”, “dentist” or “other HCW”, along with being enrolled as “IMSS employee” were defined as HCWs. Other IMSS employees with entries of different occupations from the ones previously mentioned, were reclassified as “other HCW”. The remainder of patients who did not satisfied the above-mentioned criteria were defined as GP.
Additional categories were assigned within the physician subgroup by hierarchy position and medical specialty. The former divides the patient into three groups: “attending physician”, “resident physician” and “intern”. In the latter, groups by medical specialty were classified by combining attending physicians and residents from the same area, including “anesthesiology”; “surgery”; “OB-GYN”; “internal medicine”; “primary care medicine”, which includes family medicine and general practitioners; “emergency medicine”; and “other specialties”, which includes physicians in executive positions, intensive care medicine, orthopedics, pediatrics, occupational medicine, and physical medicine and rehabilitation.
Regarding outcomes, patients with at least one positive RT-PCR test for SARS-CoV-2 were considered confirmed COVID-19 cases and patients with a negative result were considered non-COVID-19 cases.
Multiple imputation with logistic linear regression was performed. A total of 99 imputations were created using multiple imputation under the missing at random (MAR) assumption for entries where a RT-PCR for SARS-CoV-2 result was missing. Age, gender, occupation, IMSS employee, signs and symptoms, personal medical history and contact with suspect cases were considered predictors of missingness and defined as auxiliary variables for imputation before the analysis was conducted.
The mode value from the multiple imputation was assigned to registries with missing information, obtaining the following two sets of data: the complete-case analysis, excluding participants without a RT-PCR result (Analysis 1) and an alternative data set incorporating multiple imputation data including all of the patients (Analysis 2).
For the analysis of the relationship between HCW and COVID-19 case status, crude prevalence odds ratios (POR) were calculated with 95% confidence intervals (95%CI) and the χ2 test was used in the bivariate analysis, in addition to Yates correction. The Mantel-Haenszel test was used to control for confounding, stratifying by age, gender, and history of chronic disease, as no other demographic data was included in the database. Statistical analysis for each set of data was conducted using IBM SPSS Statistics 25 and Stata 15. Statistical significance was considered as a P-value < 0.05. An alternative statistical analysis using Rubin's rules for pooling multiple imputation results and binomial logistic regression to estimate the effect size of being a HCW and acquiring COVID-19 is included in the following link: http://ijms.info/IJMS/article/view/625/Supplementary_Material
From a total of 10,216 entries in the SINOLAVE registry, data from 6,256 patients was analyzed after eliminating 3,960 cases that failed to meet the inclusion criteria (3,858 were records from outside of Tijuana City, 72 were repeated, and 30 had missing data, see Figure 1). Only 897 (14.33%) patients from the 6,256 included had at least one RT-PCR test for SARS-CoV-2, thus it was possible to classify them as a COVID-19 case or a non-case for Analysis 1. On the other hand, multiple imputation was performed on data from 5,359 (85.66%) subjects to complete Analysis 2, which included all the patients involved in this study.
Figure 1Flowchart of the Study.

Mean age for Analysis 1 was 45 years (SD 13), with a minimum of 0 to a maximum of 88 years of age (Table 1). Analysis 2 showcased a mean age of 39 years (SD 19), with an age range of 0 to 97 years. The most represented age group was 40 to 59 years (47.05%) in Analysis 1, and for Analysis 2 it was 16 to 39 years (52.40%). There were slightly more males than females included in both analyses, with 493 (54.96%) vs. 404 (45.04%) in Analysis 1, and 3,190 (50.99%) vs. 3,066 (49.01%) in Analysis 2, respectively. While the Analysis 2 group included 5,634 patients (90.06%) from the GP and only 622 HCWs (9.94%), the Analysis 1 group was composed of 653 members (72.80%) of the GP and 244 HCWs (27.20%). A confirmatory test was performed on 36.01% of HCW suspect cases and only 11.59% of the GP. A history of chronic disease was more common in the Analysis 1 group with 39.69%, compared to 28.84% in Analysis 2. The most prevalent chronic diseases among HCW were hypertension (17.4%), obesity (11.9%), and asthma (8.2%), whereas in the GP they were hypertension (18.1%), obesity (13.4%), and diabetes (11.6%). A similar proportion of smokers were involved in both groups with 4.1% vs. 4.7% in HCW and GP, respectively.
Table 1.Demographic Characteristics of Study Subjects.
| Analysis 1 | Analysis 2 (Multiple Imputation) | |||||
|---|---|---|---|---|---|---|
| Variables | COVID-19 case n = 558 |
COVID-19 non-case n = 339 |
Total n = 897 |
COVID-19 case n = 3,103 |
COVID-19 non-case n = 3,153 |
Total n = 6,256 |
| Gender, n (%) | ||||||
| Male | 326 (58.42) | 167 (49.26) | 493 (54.96) | 1,770 (57.04) | 1,420 (45.04) | 3,190 (50.99) |
| Female | 232 (41.58) | 172 (50.74) | 404 (45.04) | 1,333 (42.96) | 1,733 (54.96) | 3,066 (49.01) |
| Age, years (standard deviation) | ||||||
| Mean | 47 (14) | 41 (16) | 45 (16) | 42 (13) | 36 (12) | 39 (19) |
| Range | 7–87 | 0–88 | 0–88 | 0–97 | 0–91 | 0–97 |
| Age groups, n (%) | ||||||
| 0 to 5 years | 0 (0.00) | 13 (3.83) | 13 (1.45) | 2 (0.06) | 36 (1.14) | 38 (0.61) |
| 6 to 15 years | 4 (0.72) | 6 (1.77) | 10 (1.11) | 9 (0.29) | 36 (1.14) | 45 (0.72) |
| 16 to 39 years | 170 (30.47) | 142 (41.89) | 312 (34.78) | 1,329 (42.83) | 1,949 (61.81) | 3,278 (52.40) |
| 40 to 59 years | 283 (50.72) | 139 (41.00) | 422 (47.05) | 1,476 (47.57) | 1,034 (32.79) | 2,510 (40.12) |
| >60 years | 101 (18.10) | 39 (11.50) | 140 (15.61) | 287 (9.25) | 98 (3.11) | 385 (6.15) |
| Healthcare workers, n (%) | ||||||
| Yes | 136 (24.37) | 108 (31.86) | 244 (27.20) | 384 (12.38) | 238 (7.55) | 622 (9.94) |
| No | 422 (75.63) | 231 (68.14) | 653 (72.80) | 2,719 (87.62) | 2,915 (92.45) | 5,634 (90.06) |
| History of chronic disease, n (%) | ||||||
| Yes | 228 (40.86) | 128 (37.76) | 356 (39.69) | 946 (30.49) | 858 (27.21) | 1,804 (28.84) |
| No | 330 (59.14) | 211 (62.24) | 541 (60.31) | 2,157 (69.51) | 2,295 (72.79) | 4,452 (71.16) |
Of all HCWs included (Table 2), physicians represented the largest subgroup within Analysis 1 with 96 subjects (39.34%), followed by nurses and other HCWs with 80 (32.79%) and 66 (27.05%), respectively. However, nurses represented the largest subgroup among HCWs within Analysis 2 with 236 subjects (37.94%), followed by other HCWs with 208 (33.44%), and physicians with 173 (27.81%). Likewise, within the doctors' subgroup in both Analyses 1 and 2, 41 (58.57%) and 80 (63.49%) were attending physicians; 18 (25.71%) and 26 (20.63%) were residents; and 11 (15.71%) and 20 (15.87%) were interns, respectively.
Table 2.Frequency of Healthcare Workers by Category.
| Category, n (%) | Analysis 1 (n=244) | Analysis 2 (n=622) |
|---|---|---|
| Nurses | 80 (32.79) | 236 (37.94) |
| Other healthcare workersa | 66 (27.05) | 208 (33.44) |
| Physicians | 96 (39.34) | 173 (27.81) |
| Internsb | 11 (15.71)c | 20 (15.87)c |
| Residentsb | 18 (25.71)c | 26 (20.63)c |
| Attendingsb | 41 (58.57)c | 80 (63.49)c |
| Laboratory staff | 1 (0.41) | 3 (0.48) |
| Dentists | 1 (0.41) | 2 (0.32) |
From a total of 173 physicians (Table 3) it was possible to identify the area of specialty or job position of only 126 subjects (72.8%) through a hospital records search. In both sets of analyses, the specialty with the largest representation was internal medicine. However, subtracting resident physicians, that respectively account for 30.51% and 24.52% in Analyses 1 and 2, from their respective specialties showcased that interns were the largest subset among the doctors' subgroup.
Table 3.Frequency of Physicians by Declared Medical Specialty.
| Medical specialtya, n (%) | Analysis 1 (n=70) | Analysis 2 (n=126) |
|---|---|---|
| Internal medicine | 13 (18.57) | 23 (18.25) |
| Surgery | 11 (15.71) | 20 (15.87) |
| Internsb | 11 (15.71) | 20 (15.87) |
| Primary care | 8 (11.43) | 14 (11.11) |
| Emergency medicine | 9 (12.86) | 14 (11.11) |
| Gynecology & Obstetrics | 6 (8.57) | 9 (7.14) |
| Anesthesiology | 2 (2.86) | 8 (6.35) |
| Pediatricsc | 1 (1.43) | 5 (3.97) |
| Physicians in executive positionsc | 4 (5.71) | 4 (3.17) |
| Orthopedicsc | 1 (1.43) | 4 (3.17) |
| Intensive carec | 2 (2.86) | 3 (2.38) |
| Occupational medicinec | 1 (1.43) | 1 (0.79) |
| Physical medicine and rehabilitationc | 1 (1.43) | 1 (0.79) |
The association between being a HCW and a COVID-19 confirmed case was statistically significant, both in Analysis 1 (χ2=5.947, df=1, P=0.015), and Analysis 2 (χ2=40.692, df=1, P<0.001), but the direction of risk is contrary according to each analysis. In Analysis 1, the POR=0.689 (95%CI 0.511, 0.930), whilst in Analysis 2, POR=1.730 (95%CI 1.459, 2.050). The GP was used as referent for analysis. Stratifying by age group, the statistical significance of the Analysis 1 was lost (POR=0.757; 95%CI 0.551, 1.040; χ2MH=3.566, df=1, P=0.168) It was identified that only the age group of 40 to 59 years maintained a statistically significant association (POR=0.550; 95%CI 0.349, 0.869; χ2MH=6.668, df=1, P=0.010). In this same analysis, there was no change in statistical significance after adjusting by gender (POR=0.728; 95%CI 0.537, 0.986; χ2MH=3.880, df=1, P=0.049), but higher odds were observed after adjusting by history of chronic disease (POR=1.451; 95%CI 1.075, 1.956; χ2MH=5.967, df=1, P=0.015). A slight increase in size effect was observed in Analysis 2 after adjusting by age group (POR=1.857; 95%CI 1.563, 2.206; χ2MH=51.050, df=1, P<0.001) and gender (POR=1.897; 95%CI 1.596, 2.254; χ2MH=53.552, df=1, P<0.001), whereas adjusting by history of chronic disease rendered lower odds (POR=0.578; 95%CI 0.488, 0.685; χ2MH=40.692, df=1, P<0.001).
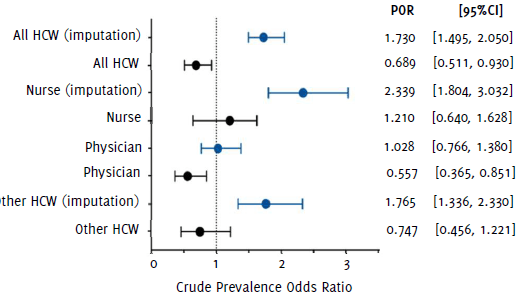
Nurses were the HCW subgroup with the highest odds of acquiring COVID-19 (Figure 2), with a POR=2.339 (95%CI 1.804, 3.032) compared to the GP in Analysis 2, and POR=1.210 (95%CI 0.640, 1.628) in Analysis 1. In addition, other HCWs had a POR=1.765 (95%CI 1.336, 2.330) in Analysis 2, whereas in Analysis 1 this was not statistically significant (OR=0.689; 95%CI 0.511, 0.930). On the other hand, physicians showcased a protective factor in Analysis 1 (POR=0.557; 95%CI 0.365, 0.851) and a small excess in effect size compared to the GP in Analysis 2 (POR=1.028; 95%CI 0.766, 1.380). No change in statistical significance observed after stratifying by gender, age group, and history of chronic disease. It was not possible to estimate the association and individual risk of dentists and laboratory staff for COVID-19 given the low number of subjects in these subgroups.
Figure 2Unadjusted Prevalence Odds Ratios (POR) and 95% Confidence Intervals (95%CI) for COVID-19 According to Healthcare Worker Category.
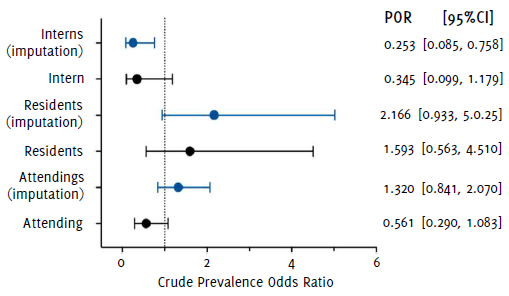
Within the different physician hierarchies (Figure 3), it was found that interns had a POR=0.345 (95%CI 0.099, 1.179) and POR=0.253 (95%CI 0.085, 0.758) in Analyses 1 and 2, respectively. Meanwhile, residents had a higher likelihood of acquiring COVID-19 than the GP in both analyses (Analysis 1: POR=1.593; 95%CI 0.563, 4.510; Analysis 2: POR=2.166; 95%CI 0.933, 5.025). On the other hand, attending physicians showcased a POR=0.561 (95%CI 0.290, 1.083) in Analysis 1, and POR=1.320 (95%CI 0.841, 2.070) in Analysis 2. Adjusting by gender, age group and history of chronic disease showed no difference in statistical significance.
Figure 3Unadjusted Prevalence Odds Ratios (POR) and 95% Confidence Intervals (95%CI) for COVID-19 According to Medical Hierarchy.
Further analysis was conducted to estimate the risk attached to each medical specialty included in this study compared to that of the cluster of physicians (Figure 4). It was observed that emergency medicine had the highest odds for contracting COVID-19 among medical specialties (Analysis 1: POR=8.828; 95%CI 1.040, 74.934; Analysis 2: POR=4.071; 95%CI 1.090, 15.208), followed by anesthesiology (Analysis 1: POR=1.943; 95%CI 1.452, 2.447; Analysis 2: POR=2.806; 95%CI 0.544, 14.466). Surgeons (Analysis 1: POR=1.084; 95%CI 0.298, 3.946; Analysis 2: POR=1.963; 95%CI 0.734, 5.247) and primary care physicians (Analysis 1: POR=1.563; 95%CI 0.343, 7.112; Analysis 2: POR=1.200; 95%CI 0.391, 3.680) also showed increased odds compared to that of all doctors. The internal medicine specialists had a possible protective factor (Analysis 1: POR=0.71; 95%CI 0.215, 2.407; Analysis 2: POR=0.722; 95%CI 0.313, 1.906). Likewise, all other medical specialties, which for this analysis included intensive care physicians, pediatricians, and physicians in executive positions had a lower likelihood of acquiring COVID-19 (Analysis 1: POR=0.629; 95%CI 0.205, 1.929; Analysis 2: POR=0.156; 95%CI 0.017, 1.048). On the other hand, OB-GYN was shown to have conflicting effect size estimates (Analysis 1: POR=0.822; 95%CI 0.165, 4.706; Analysis 2: POR=1.111; 95%CI 0.284, 4.343).
Figure 4Unadjusted Prevalence Odds Ratios (POR) and 95% Confidence Intervals (95%CI) for COVID-19 by Medical Specialty.

In this study, HCWs had 73% higher odds of acquiring COVID-19 than the GP. A disparity in the number of COVID-19 confirmatory tests was observed, since the HCW cluster was tested at least three times more (36.01%) than the GP (11.59%). Therefore, multiple imputation was performed to reduce the bias generated by the lack of confirmatory test results. Comparing between HCW categories, nurses were identified as the group with highest likelihood of acquiring COVID-19, with nearly double the odds of the GP. Conversely, the physician subgroup showcased a statistically significant protective factor in one of the analyses. However, using Analysis 2, it demonstrated only an additional 2.8% increase in odds from the GP, without statistical significance. Analyzing the physicians cluster by hierarchy, the group with the largest effect size estimate was resident physicians, with approximately 50% to 60% higher odds than GP in both analyses, but neither were statistically significant. On the contrary, interns showcased a potential protective factor compared to the GP. Finally, emergency medicine held the largest effect size among the medical specialties included in this study. A four- to eight-fold increase in odds compared to the all the other medical specialties was observed, and although statistically significant, wide confidence intervals were estimated. Anesthesiology followed as the second medical specialty with the highest likelihood of infection, by nearly double the estimate, but also with wide confidence intervals. In contrast, internal medicine posed a possible protective factor, with a close to 30% decreased likelihood of contracting COVID-19 than the rest of physicians; however, this finding was not statistically significant in either analysis.
Among all confirmed cases of COVID-19, HCWs represent nearly a quarter of the patients in Analysis 1 and only 7.55% in Analysis 2. In this study, HCWs were demonstrated to have roughly 73% higher odds of acquiring COVID-19 than the GP. This can be explained by HCW having direct or indirect contact with multiple patients and their surroundings, sometimes in confined areas.17,19 Thus, HCW may experience a greater exposure to the virus, both chronologically and quantitively, than the GP. Even though infection prevention protocols were established according to HCW categories and tasks from the start of the pandemic, these measures were mostly focused on droplet and contact transmissions.20 However, as recently reported, SARS-CoV-2 transmissibility can be heterogeneous21,22 and the ability to appropriately don and doff PPE varies widely between each individual worker and by level of training.1,23 Age was found to be a possible confounding factor in one of the analyses, this can be attributed to the fact that most HCW included in this study were in the age group of 40 to 59 years. Although this phenomenon was not seen in Analysis 2. Therefore, being an HCW —independently of category, despite the use of PPE, and other protective measures—represents a major risk of acquiring COVID-19. Although it was not possible to calculate the effect size estimate for every individual category included under the term HCW, nurses were identified as the group with the highest likelihood for acquiring COVID-19. This phenomenon has been previously described by Chen et al.24 during the 2009 influenza pandemic in Singapore, while other authors25 have found that nurses have a greater COVID-19 mortality rate compared to physicians in Italy, Brazil, Spain and France. This could be attributed to multiple factors, such as the type and length of interventions carried out by nurses and having more frequent and closer contact with patients for extended periods of time compared to, for example, physicians.26,27 Therefore, they are subjected to a greater exposure than the rest of the healthcare workforce. Additionally, it should be considered that nurses are the largest group of all the HCWs in this study population. Because of this, they may also have higher probabilities of coming into contact with infected colleagues in the workplace. On the other hand, physicians were subjected to a smaller effect size, and even appeared to have a degree of protection in Analysis 1. This could be explained considering the diversity within medical specialties, including the heterogeneity of procedures they perform and the PPE recommended for each group. A similar situation emerged when analyzing the odds of other HCWs, which included a vast range of job positions such as physicians in executive roles, social workers, receptionists, stretcher-bearers, ambulance drivers, cleaning staff, among others; each one of them with a different level of occupational exposure and PPE usage requirements.7,28
Comparing hierarchy roles among physicians, residents were the group of doctors with the highest odds of acquiring COVID-19 compared to the GP. Although resident physicians essentially partake in the same activities as their attendings, the workload is not comparable. The long working hours and greater frequency of contact with patients29,30 appears to increase the risk of exposure to infected patients in this group. Moreover, residency training for physicians is a well-established stressful experience, which may contribute to a compromised immune system.31,32 Conversely, interns usually execute tasks of a slightly lesser complexity but under the same working conditions as residents. However, in Mexico they are still considered medical students and therefore most of them were withdrawn from COVID-19 high-risk areas33 and, in addition to being younger than the rest of physicians, this could have contributed to lower odds of contracting COVID-19 for this group.
Analyzing the differences in effect size estimates between medical specialties, emergency medicine physicians had the highest odds for COVID-19. This coincides with the results published by Whiteside et al.,34 in which emergency department and primary care personnel infection risk was greater than that of other areas. This could be explained considering that emergency rooms are primary points of entry to any other department in most hospitals. Despite the implementation of entry-point filters for patients with respiratory symptoms and COVID-19 suspect cases, emergency physicians are still exposed to many patients seeking urgent medical attention for other reasons while possibly being asymptomatic carriers of SARS-CoV-2,35 and even perform resuscitation maneuvers in severely ill patients, some of whom could be potential COVID-19 cases. Moreover, patients gathering in emergency rooms is commonplace in Mexico, compromising the implementation of infection control and prevention measures required to limit disease transmission. Not surprisingly, the second medical specialty with highest odds was anesthesiology, as they perform aerosol-generating procedures on a regular basis,36 and consequently have a greater exposure to viral particles. In contrast, other medical specialties showcased a protective factor, such as internal medicine and OB-GYN, although neither had statistically significant results. However, it is necessary to further investigate if different, or even more stringent measures—such as indiscriminate use of PPE and implementation of multiple filter systems for patients—are being taken that could explain this phenomenon.
The limitations of this study are inherent to the design itself, considering that the data used was not specifically generated with the intention of answering our research question. Errors in categorization could have been made due to not having complete information on the occupation from all participants. Likewise, lack of information about HCW type of contact with patients, working hours, and frequency of exposure did not allow for further analysis to meaningfully compare different patterns between HCW categories. These results are based on data from a public healthcare system in one city in northern Mexico and thus is not necessarily internationally generalizable. It should be noted that POR is not an estimation of risk and therefore these results are to be cautiously interpreted, as they could overestimate the effect size if an approximation to risk is to be inferred. Multiple imputation helped avoid further reduction of our study population and mitigated the bias from missing data. Nevertheless, using this method for analysis showcased some opposing results that could be explained by a number of factors. Primarily, multiple imputation using the MAR assumption implies a random distribution of attributes under the premise that missing data depends on the observed data and not on the values of the missing data, whereas RT-PCR results in Analysis 1 were obtained by testing individuals according to clinical judgement and hospital policies and resources. As a result, characteristics such as the auxiliary variables used for imputation contribute to predict missing data, but with limitations such as complete medical records and individual hospital policies and procedures for testing were not included in the database. Therefore, the distribution of cases could differ from actuality in both analyses. Likewise, results regarding medical specialties should be interpreted cautiously, as the number of participants included was low, resulting in wide confidence intervals. Finally, our study also takes into consideration the non-occupational risk to which HCWs are also exposed to outside the workplace, for instance the analyses used the GP as referent.
In this cross-sectional database study, it was demonstrated that HCWs have higher odds of acquiring COVID-19 than the GP among IMSS users in Tijuana, Mexico. Nurses were the HCW group with the highest likelihood of acquiring SARS-CoV-2 infection. Regarding physician hierarchy, residents had the biggest effect estimate. On the other hand, interns, who were removed from COVID-19 high-risk areas, showcased a protective factor. Moreover, among medical specialties included in this study, emergency medicine and anesthesiology have the highest odds for contracting COVID-19, likely owing to the frequent execution of aerosol-generating procedures. In addition, medical specialties assumed to be more exposed to confirmed COVID-19 cases, such as internal medicine, or departments where more thorough infection control practices are systematically applied, such as OB-GYN, had a possible protective factor. Complementary studies are required to confirm our findings including a bigger and more open population, and even a follow-up of this study population, considering risk factors associated with each HCW category. It is essential to perform local and nation-wide research in order for health authorities to endorse evidence-based preventive protocols aimed at protecting and supporting the workforce that is currently sustaining healthcare systems during the crisis.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: JY, CP, SR & MR. Methodology: JY, CP & SR. Validation: JY, GG & MR. Formal Analysis: SR. Investigation: JY, CP & SR. Resources: JY & CP Data Curation & Writing – Original Draft: JY, CP, SR & GG. Writing – Review & Editing: JY, CP, GG & MR. Visualization: JY, GG. Supervision: JY, CP. Project Administration: JY, CP, & MR.
1. Bhagavathula AS, Aldhaleei WA, Rahmani J, Mahabadi MA, Bandari DK. Knowledge and Perceptions of COVID-19 Among Health Care Workers: Cross-Sectional Study. J Med Internet Res. 2020 Apr 30;6(2):e19160.
2. Koh D. Occupational risks for COVID-19 infection. Occup Med (Lond). 2020 Mar 12;70(1):3-5
3. Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, et al. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano. 2020 Apr 28;14(4):3822-35.
4. Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China - Key questions for impact assessment. N Engl J Med. 2020 Feb 20;382(8):692-4.
5. Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk Factors of Healthcare Workers with Corona Virus Disease 2019: A Retrospective Cohort Study in a Designated Hospital of Wuhan in China. Clin Infect Dis. 2020 Nov 19;71(16):2218-21.
6. Hunter E, Price DA, Schim van der Loeff I, Baker KF, Lendrem D, Lendrem C, et al. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020 May 2;395(10234):e77-78
7. Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med. 2020 Mar;8(3):e13.
8. World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease2019 (COVID-19). Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Last updated Feb 24, 2020; cited Nov 30, 2020.
9. García-Méndez N, Lagarda Cuevas J, Otzen T, Manterola C. Anesthesiologists and the High Risk of Exposure to COVID-19. Anesth Analg. 2020 Aug;131(2):e92-93.
10. Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020 May;493(5):489-93.
11. Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239-42.
12. Hamed E, Abd Elhamid M, Alemrayat B. Suspected cases of COVID-19: study protocol for reporting characteristics and the outcomes. Fam Med Community Heal. 2020 Apr;8(2):e000400.
13. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is fecal-oral transmisssion of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020 Apr;5(4):335-7.
14. Dijkhuizen LGM, Gelderman HT, Dujist WLJM. Review: The safe handling of a corpse (suspected) with COVID-19. J Forensic Leg Med. 2020 Jul;73:101999.
15. Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020 May;99(5):565-8.
16. Huh S. How to train health personnel to protect themselves from SARS-CoV-2 (novel coronavirus) infection when caring for a patient or suspected case. J Educ Eval Health Prof. 2020 Jan;17:10.
17. Zhang Z, Liu S, Xiang M, Li S, Zhao D, Huang C, et al. Protecting healthcare personnel from 2019-nCoV infection risks: lessons and suggestions. Front Med. 2020 Apr;14(2):229-31.
18. Gobierno de Mexico. Versión estenográfica. Conferencia de prensa. Informe diario sobre coronavirus COVID-19 en México 24-03-2020. Available from: https://www.gob.mx/presidencia/articulos/version-estenografica-conferencia-de-prensa-informe-diario-sobre-coronavirus-covid-19-en-mexico-240978?fbclid=IwAR0eb_SC3RN2f921tq-qw79EFSQIfpPNKzVHF5qsR0zTfBWtvccyvoM9rTg. Last updated Mar 24, 2020; cited May 26, 2020.
19. Wilder-Smith A, Teleman MD, Heng BH, Earnest A, Ling AE, Leo YS. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005 Jul;11(7):1142-5.
20. Secretaría de Salud. Lineamiento técnico de uso y manejo del equipo de protección personal ante la pandemia por COVID-19. Available from: https://coronavirus.gob.mx/wp-content/uploads/2020/05/Lineamiento_uso_manejo_EPP_COVID-19.pdf. Last updated May 12, 2020; cited May 26, 2020.
21. He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 May;26(5):672-5.
22. Gandhi M, Yokoe DS, Havlir D V. Asymptomatic Transmission, the Achilles' Heel of Current Strategies to Control Covid-19. N Engl J Med. 2020 May 28;382(22):2158-60.
23. Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020 May;105(1):100-101.
24. Chen MIC, Lee VJM, Barr I, Lin C, Goh R, Lee C, et al. Risk factors for pandemic (H1N1) 2009 virus seroconversion among hospital staff, Singapore. Emerg Infect Dis. 2010 Oct;16(10):1554-61.
25. Jackson D, Anders R, Padula W V, Daly J, Davidson PM. Vulnerability of nurse and physicians with COVID-19: Monitoring and surveillance needed. J Clin Nurs. 2020 Oct;29(19-20):3584-87.
26. Bernard H, Fischer R, Mikolajczyk RT, Kretzschmar M, Wildner M. Nurses' contacts and potential for infectious disease transmission. Emerg Infect Dis. 2009 Sep;15(9):1438-44.
27. Huang L, Lin G, Tang L, Yu L, Zhou Z. Special attention to nurses' protection during the COVID-19 epidemic. Crit Care. 2020 Mar 27;24(1):120.
28. Wei L, Fang T, Fang L-Q, de Vlas SJ, Ma H-J, Zhou J-P, et al. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Trop Med Int Heal. 2009 Oct 7;14(1):52-9.
29. Prieto-Miranda SE, Jiménez-Bernardino CA, Cázares-Ramírez G, De Vera-Haro MJ, Esparza-Pérez RI. [Working hours and their repercussions on resident doctors in a second level hospital]. 2015 Nov;31:669-79. Esp
30. Acosta-Fernández M, Aguilera-Velasco MA, Pozos-Radillo BE, Torres-López TM, Parra Osorio L. [Experiences of Mexican resident physicians during their first year of education]. Inv Ed Med. 2017 Nov 8;6(23):169-79. Esp
31. Weiss P, Kryger M, Knauert M. Impact of extended duty hours on medical trainees. Sleep Health. 2016 Dec;2(4):309-15.
32. Ridout KK, Ridout SJ, Guille C, Mata DA, Akil H, Sen S. Physician-Training Stress and Accelerated Cellular Aging. Biol Psychiatry. 2019 Nov 1;86(9):725-30.
33. Dirección General de Calidad y Educación en Salud. Statement No DGCES-DG-0436-2020 Undergraduate Intern Physicians During Phase II of contingency due to COVID-19 pandemic. Mexico City; Apr 7, 2020. Available from: https://www.amfem.edu.mx/index.php/acerca/comunicados?start=3. Last updated Apr 7, 2020; cited May 26, 2020.
34. Whiteside T, Kane E, Aljohani B, Alsamman M, Pormand A. Redesigning emergency department operations amidst a viral pandemic. Am J Emerg Med. 2020 Jul;38(7):1448-53.
35. Canova V, Lederer Schlpfer H, Piso RJ, Droll A, Fenner L, Hoffmann T, et al. Transmission risk of SARS-CoV-2 to healthcare workers – observational results of a primary care hospital contact tracing. Swiss Med Wkly. 2020 Apr 25;150:w20257.
36. Weissman DN, de Perio MA, Radonovich Jr LL. COVID-19 and risks posed to personnel during endotracheal intubation. JAMA. 2020 May 26;323(20):2027-8.
José Adrián Yamamoto-Moreno, 1 MD, Universidad Autónoma de Baja California, Facultad de Medicina y Psicología; Instituto Mexicano del Seguro Social, H. Gineco-Obstetricia y Unidad de Medicina Familiar No. 7, Tijuana, México
Cecilia Pineda-Aguilar, 1 MD, Universidad Autónoma de Baja California, Facultad de Medicina y Psicología; Instituto Mexicano del Seguro Social, H. Gineco-Obstetricia y Unidad de Medicina Familiar No. 7, Tijuana, México
Samuel Ruiz-Pérez, 2 Medical student, Universidad Autónoma de Baja California, Facultad de Medicina y Psicología, Tijuana, Mexico
Gloria Liliana Gortarez-Quintana, 2 Medical student, Universidad Autónoma de Baja California, Facultad de Medicina y Psicología, Tijuana, Mexico
Marco Antonio Ruiz-Dorado, 3 Internal medicine specialist, Instituto Mexicano del Seguro Social, Hospital de Gineco-Obstetricia y Unidad de Medicina Familiar No. 7, Tijuana, México
About the Author: José Adrián is a recently graduated physician from a 7-year program at Universidad Autónoma de Baja California in México. He was awarded the Carlos Slim Foundation scholarship for excellence in Medicine and served as National Officer of Medical Publications in Asociación Mexicana de Médicos en Formación (AMMEF) from 2019-2020.
Correspondence: José Adrián Yamamoto-Moreno. Address: Tijuana, México. Email: adrian.yamamoto@uabc.edu.mx
Editor: Francisco J. Bonilla-Escobar Copyeditor: Nikoleta Tellios Proofreader: Ciara Egan Layout Editor: Sushil Dahal
Cite as: Yamamoto-Moreno JA, Pineda-Aguilar C, Ruiz-Pérez S, Gortarez-Quintana GL, Ruiz-Dorado MA. SARS-CoV-2 Infection Among Healthcare Workers in Tijuana, Mexico: A cross-sectional study. Int J Med Students. 2020 Sep-Dec;8(3):220-5.
Copyright © 2020 José Adrián Yamamoto-Moreno, Cecilia Pineda-Aguilar, Samuel Ruiz-Pérez, Gloria Liliana Gortarez-Quintana, Marco Antonio Ruiz-Dorado
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 8, NUMBER 3, December 2020