Control group diagnoses
Bojan Popchanovski1, Margareta Balabanova-Stefanova2
doi: http://dx.doi.org/10.5195/ijms.2019.438
Volume 7, Number 3: 62-65
Received 20 10 2019: Rev-recd 27 10 2019: Rev-recd 12 12 2019: Accepted 16 12 2019
ABSTRACT
Background:Psoriasis is a chronic cutaneous T-cell mediated disease, which has been associated with many comorbidities, such as metabolic disorders. Specific abnormalities include dyslipidemia, insulin resistance, obesity, and metabolic syndrome, many of which are themselves risk factors for other diseases. The goal of this study was to evaluate the presence of dyslipidemia and hyperglycemia in patients with psoriasis.
Methods:We compared 48 inpatients with plaque psoriasis aged 29-79, hospitalized between March 2018 and February 2019, to 48 age- and gender-matched controls. We evaluated dyslipidemia and hyperglycemia using enzymatic methods as part of a standard blood test, or medication history indicative of ongoing treatment of dyslipidemia and/or hyperglycemia. Hypertension was evaluated by registering blood pressure greater than 140/90 mmHg or ongoing antihypertensive treatment. Smoking habits were also noted.
Results:There were statistically significant differences between psoriasis patients and controls for elevated total cholesterol (p=0,028), elevated LDL (p=0,015), hypertriglyceridemia (p=0,006), and hyperglycemia (p=0,021). The two groups had statistically insignificant differences for lowered HDL (p=0,084), hypertension (p=1), and smoking (p=0,836).
Conclusion:Hypertriglyceridemia, hyperglycemia, and elevated LDL cholesterol were found to be more prevalent in the group containing psoriatic patients compared to the control group. This indicates that further investigation of metabolic abnormalities should be conducted in psoriatic patients which could greatly benefit from early treatment of the aforementioned underlying conditions.
Keywords: Psoriasis; Inpatients; Metabolic syndrome; Dyslipidemias; Hyperglycemia (Source: MeSH-NLM).
Psoriasis is a chronic immune-mediated skin disorder, with a prevalence of 2%.1 TNF-α, IFN-α, IL-23 and Th-17 cells play an important role in the pathogenesis of psoriasis.2 Recent evidence suggests that metabolic abnormalities are present in the milieu of chronic inflammation, as in the case of rheumatological diseases.3 Chronic inflammation is thought to cause cytokine-induced changes in glucose and lipoprotein metabolism,4 alluding to a similar situation which happens in the case of insulin resistance caused by cytokines secreted by adipose tissue.5
The amount of data on the effect of psoriasis on metabolism is increasing, but various results have been reported. For triglyceride levels, there are studies that found increased levels6–10 as well as statistically insignificant changes.6, 11, 12 There are studies that associated psoriasis with higher,12, 13 and others with normal LDL cholesterol levels.6, 8, 11, 14–16 As for HDL cholesterol, there are studies that associated psoriasis with lower6, 13, 15, 16 HDL levels, and studies that did not make that association.8, 11, 12, 14, 17, 18 Correlation was reported between psoriasis and diabetes mellitus in some studies9–11, 19, 20 and in other studies no such correlation was made.6, 7, 14, 17 Results on hypertension and psoriasis were also conflicting, as there are studies that established a link9, 10, 19, 20 and studies that did not.7, 15
Quantitative and qualitative changes in lipoprotein metabolism, caused by chronic inflammation, may be of potential clinical significance in patients with a high risk of cardiovascular comorbidity. This study was conducted to examine the correlation between psoriasis and abnormal glucose and lipid metabolism.
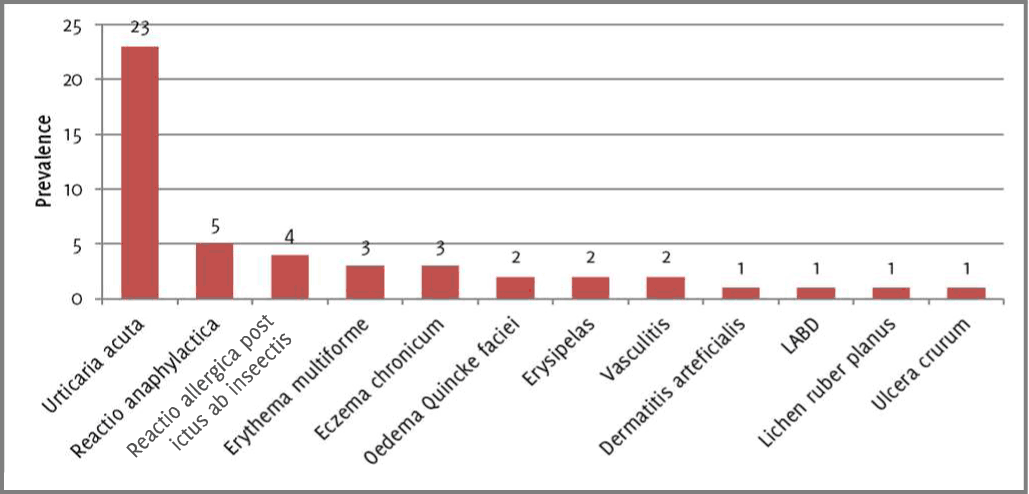
This retrospective study included 48 inpatients (27 males, 21 females) with psoriasis vulgaris (plaque and nummularis type) aged 29-79, hospitalized in the University Clinic of Dermatology at the Medical Faculty in Skopje, between March 2018 and February 2019. Data was derived from the clinic's inpatient medical records. Psoriatic inpatients that had pustular psoriasis, psoriatic arthritis, erythrodermia, prior systemic treatment for psoriasis, concomitant tumors, chronic lung, heart, kidney and rheumatological diseases were excluded from the study. These 48 inpatients were paired with another 48 inpatients, matched for age (±1 year) and gender, hospitalized within the same timeframe, and on the same clinic. The exclusion criteria were the same for this group. The diagnoses of the control group inpatients were the following (Figure 1): Urticaria acuta (23), Reactio anaphylactica (5), Reactio allergica post ictus ab insectis (4), Erythema multiforme (3), Eczema chronicum (3), Oedema Quincke faciei (2), Erysipelas (2), Vasculitis (2), Dermatitis arteficialis (1), LABD (1), Lichen ruber planus (1), and Ulcera crurum (1).
Figure 1Control group diagnoses

The variables of interest were triglyceridemia, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, glycemia, blood pressure, and smoking habits. Lipid parameters and blood pressure were evaluated according to cutoff values recommended by the National Cholesterol Education Program's Adult Treatment Panel III (ATP III), or ongoing antilipidemic and/or antihypertensive treatment according to the patient's medical history. These cutoff values were: 1.7 mmol/L for triglycerides, 3.3 mmol/L for LDL cholesterol, 1.0 mmol/L for HDL cholesterol, 140/90 mmHg for blood pressure. Glycemia was evaluated using the cutoff value of 6.1 mmol/L, recommended by the World Health Organization21, or ongoing antidiabetic treatment. Smoking habits were evaluated using two categories: patients who are non-smokers, and patients who are currently smoking or have smoked in the past. Glycemia and lipid parameters were measured using enzymatic methods. Blood pressure was measured with a standard mercury sphygmomanometer. RStudio was used to perform a Student's t-test and to calculate the odds ratio with 95% confidence interval.
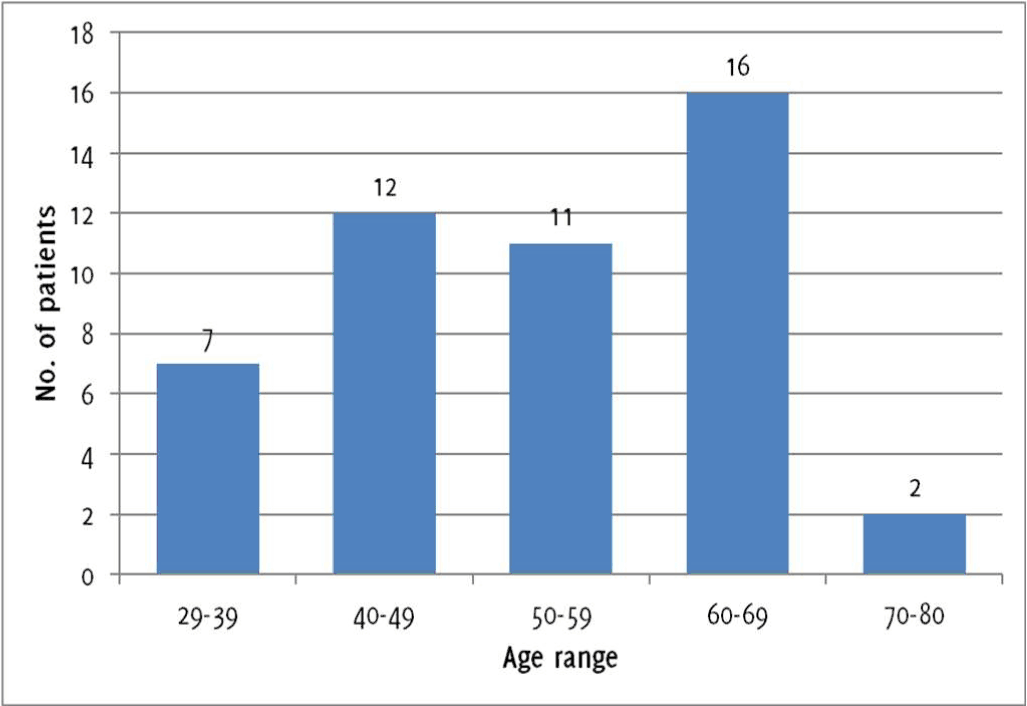
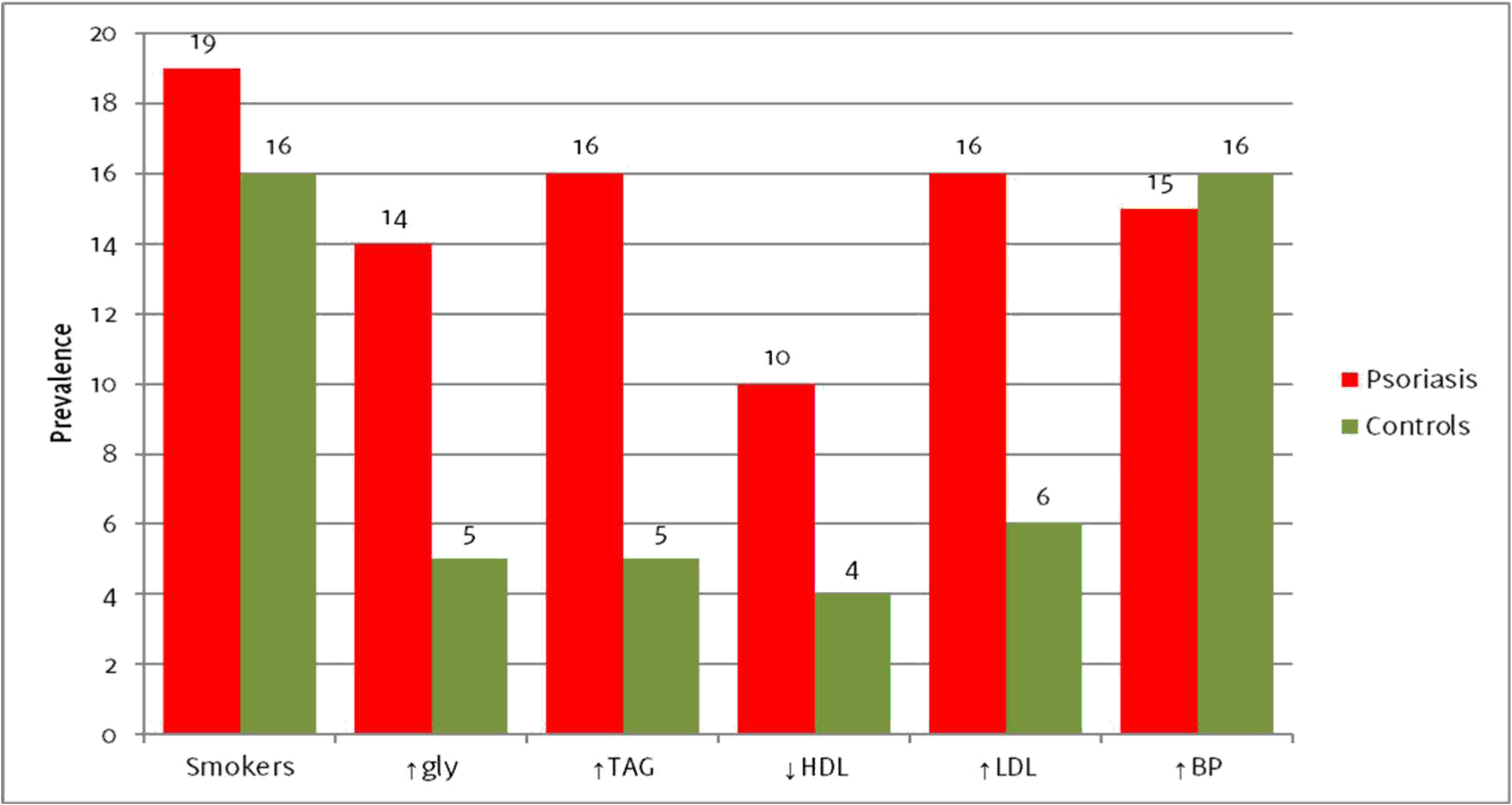
Among the 48 psoriatic patients, 7 were aged between 29-39, 12 were aged between 40-49, 11 were aged between 50-59, 16 were aged between 60-69, and 2 were aged between 70-80, as showed in Figure 2. Identical distributions were present in the control group. In the psoriasis group, 14 patients (29,17%) had hyperglycemia, compared to 5 (10,42%) in the control group (p=0,021, OR 3.54, 95%CI 1.16-10.81). Hypertriglyceridemia was noted in 16 psoriatic patients (33,33%), and in 5 patients (10,42%) in the control group (p=0,006, OR 4.30, 95%CI 1.43-12.96). LDL cholesterol was increased in 16 psoriatic patients (33,33%), compared to 6 (12,5%) control patients, (p=0,015, OR 2.14, 95%CI 0.50-9.12). The differences between the psoriasis group and the control group were statistically insignificant for the remaining parameters. Ten (20,83%) psoriatic inpatients had lowered HDL cholesterol, compared to 4 (8,33%) control patients (p=0,084, OR 2.89, 95%CI 0.84-9.98). In the psoriasis group, 19 patients (39,58%) reported to have smoked or were current smokers, compared to 16 (33,33%) in the control group (p=0,836, OR 1.31, 95%CI 0.57-3.02). Finally, 16 patients in each group were found to have hypertension (p=1, OR 1.00, 95%CI 0.43-2.34). A summary of the results is given in Table 1 and Figure 3.
Figure 2Age of participants
Summary of results
Associated factors with psoriasis.
| Parameter | Psoriasis | Controls | p-value | OR (95% CI) |
|---|---|---|---|---|
| Mean age | 52,92 | 52,73 | - | - |
| Sex (male/female) | 27/21 | 27/21 | - | - |
| Smokers | 19 | 16 | 0,836 | 1.31(0.57-3.02) |
| ↑gly | 14 | 5 | 0,021 | 3.54 (1.16-10.81) |
| ↑TAG | 16 | 5 | 0,006 | 4.30 (1.43-12.96) |
| ↓HDL | 10 | 4 | 0,084 | 2.89 (0.84-9.98) |
| ↑LDL | 16 | 6 | 0,015 | 2.14 (0.50-9.12) |
| ↑BP | 15 | 16 | 1 | 1.00(0.43-2.34) |
Despite the conflicting findings of the current body of research on this topic, there is a complex pathophysiological explanation for the quantitative and qualitative changes in the case of rheumatological diseases, which may also be true for psoriasis. Proinflammatory cytokines released during the course of these diseases change many aspects of lipid metabolism, such as increased VLDL and triglyceride levels via increased hepatic fatty acid synthesis, decreased hepatic fatty acid oxidation and increased adipose tissue lipolysis. This ultimately contributes to the increase of triglyceride content in LDL and HDL particles, which subsequently leads to the formation of small dense LDL (sdLDL) particles. These particles are more atherogenic as a result of their high susceptibility to oxidation, high affinity for intra-arterial proteoglycans, and decreased clearance due to decreased affinity for LDL receptors. Additionally, lipoprotein lipase (LPL) activity is reduced, which further reduces the clearance of LDL particles.3
HDL particles are also subject to change in an inflammatory milieu, which equates to reverse cholesterol transport being severely impacted as a result. Apo A-1 clearance is increased due to decreased synthesis and increased breakdown in the kidneys, which both lead to lower affinity of Apo A-1 for HDL particles. Serum amyloid A (SAA), an acute phase protein generated during inflammation, binds to HDL particles, which lowers the affinity of Apo A-1 for its receptor, and increases the clearance of HDL particles. Cholesterol ester transfer protein (CETP) and lecithin-cholesterol acyltransferase (LCAT) levels are decreased, which lead to decreased cholesterol transport from HDL particles and decreased cholesterol ester formation, respectively. Certain phospholipid and cholesterol membrane transport proteins, such as ABCA1, ABCG1, and SR-B1, have reduced activity, which contributes to decreased hepatocyte uptake and decreased efflux from macrophages. Finally, lipoprotein (a) is generated, which has a high atherogenic potential.3 This evidence of qualitative changes in lipoproteins suggests that perceived normal lipid levels may not be enough to exclude abnormalities in lipid metabolism.
The inflammatory pathogenesis of psoriasis suggests that, skin and joint lesions aside, many more less visible metabolic effects may be present. Psoriasis causes slight but clinically actionable alterations in certain metabolic parameters, which are relevant in terms of cardiovascular comorbidity.
This study could be improved by increasing the sample size to increase the accuracy of the data and to narrow down the confidence intervals. An important drawback represents its retrospective design. The data gathered were only the parameters that are measured during routine examination in our clinic. Ethnicity is also one of these parameters not registered routinely, which may influence the prevalence, age of onset, disease course, and further changes to metabolic parameters. Additional useful parameters such as Psoriasis Area and Severity Index (PASI) and hsCRP, to determine the extensiveness of the psoriatic lesions and the cardiovascular risk, respectively, could be measured and tested more appropriately in a case-control scenario.
Another aspect not covered in this study is disease progress. Our results are only indicative of one point in time, and the history of disease progress and treatment for each individual patient is unknown. Five of the previously mentioned studies stated that their objective was to determine the prevalence specifically of metabolic syndrome in psoriatic patients7, 9, 10, 14, 17, 22. Four of them associated psoriasis with metabolic syndrome,7, 9, 10, 17 and one found no such link14. One of these previously mentioned studies established a dose-response relationship between the severity of psoriasis and the prevalence of metabolic syndrome10, while another disproved that22. One meta-analysis, taking 12 studies into account, also established a dose-response relationship4. These diverse findings pertaining to the metabolic syndrome, combined with the diverse aforementioned results on individual metabolic parameters, indicate that many other factors, such as age of onset, duration, disease severity, and treatment, may play a role in terms of the order in which metabolic changes appear, and in the way they evolve over time. Consequently, we think that the next step in psoriasis research could be the effect of the dynamics of the PASI score and BMI over time and their ability to predict inapparent but forthcoming metabolic changes.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: BP. Methodology: BP. Validation: BP. Formal Analysis: BP. Data Curation: BP. Investigation: BP. Writing – Original Draft: BP. Writing – Review & Editing: BP. Visualization: BP. Supervision: MBS. Project Administration: MBS.
1.Christophers E. Psoriasis–epidemiology and clinical spectrum. Clin Exp Dermatol. 2001 Jun;26 (4):314–20.
2.Boehncke W-H, Schön MP. Psoriasis. Lancet. 2015Sep5;386 (9997):983–94.
3.Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins: MDText. com, Inc., South Dartmouth (MA); 2000.
4.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: A systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013 Apr;68 (4):654–662
5.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006 Jul;116 (7):1793–801.
6.Reynoso-von Drateln C, Martínez-Abundis E, Balcázar-Muñoz BR, Bustos-Saldaña R, González-Ortiz M. Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. J Am Acad Dermatol. 2003 Jun;48 (6):882–5.
7.Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol. 2018 Jan-Feb;36 (1):21–28.
8.Asha K, Singal A, Sharma SB, Arora VK, Aggarwal A. Dyslipidaemia & oxidative stress in patients of psoriasis: Emerging cardiovascular risk factors. Indian J Med Res. 2017 Dec;146 (6):708–713
9.Nisa N, Qazi M. Prevalence of metabolic syndrome in patients with psoriasis. Indian J Dermatol Venereol Leprol. 2010 Nov-Dec;76 (6):662–5.
10.Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN. Prevalence of Metabolic Syndrome in Patients with Psoriasis: A Population-Based Study in the United Kingdom. J Invest Dermatol. 2012 Mar;132(3 Pt 1):556–62.
11.Seçkin D, Tokgözoğlu L, Akkaya S. Are lipoprotein profile and lipoprotein (a) levels altered in men with psoriasis? J Am Acad Dermatol. 1994 Sep;31(3 Pt 1):445–9.
12.Piskin S, Gurkok F, Ekuklu G, Senol M. Serum Lipid Levels in Psoriasis. Yonsei Med J. 2003 Feb;44 (1):24–6.
13.Solak Tekin N, Tekin IO, Barut F, Yilmaz Sipahi E. Accumulation of Oxidized Low-Density Lipoprotein in Psoriatic Skin and Changes of Plasma Lipid Levels in Psoriatic Patients. Mediators Inflamm. 2007, 2007:78454.
14.Damevska K, Neloska L, Gocev G, Mihova M. Metabolic syndrome in untreated patients with psoriasis: case-control study. J Dtsch Dermatol Ges. 2013 Dec;11 (12):1169–75.
15.Miao C, Li J, Li Y, Zhang X. Obesity and dyslipidemia in patients with psoriasis: A case-control study. Medicine (Baltimore). 2019 Aug;98 (31):e16323.
16.Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta. 2001 Jan;303 (1-2):33–9.
17.Itani S, Arabi A, Harb D, Hamzeh D, Kibbi A-G. High prevalence of metabolic syndrome in patients with psoriasis in Lebanon: a prospective study. Int J Dermatol. 2016 Apr;55 (4):390–5.
18.Seishima M, Seishima M, Mori S, Noma A. Serum lipid and apolipoprotein levels in patients with psoriasis. Br J Dermatol. 1994 Jun;130 (6):738–42.
19.Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: An experience from the Middle East. J Dermatol. 2010 Feb;37 (2):146–55.
20.Shiba M, Kato T, Izumi T, Miyamoto S, Nakane E, Haruna T. Risk of myocardial infarction in patients with psoriasis: A cross-sectional patient-population study in a Japanese hospital. J Cardiol. 2019 Apr;73 (4):276–279.
21.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. 2006.
22.Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case–control study. Br J Dermatol. 2007 Jul;157 (1):68–73
Bojan Popchanovski, 1 Medical Student, Medical Faculty, University Ss. Cyril and Methodius, Skopje, North Macedonia
Margareta Balabanova-Stefanova, 2 MD, PhD, University Clinic of Dermatology, Medical Faculty, University Ss. Cyril and Methodius, Skopje, North Macedonia
Madeleine Jemima Cox, Student Editor
Mihnea-Alexandru Găman, Editor
Correspondence: Bojan Popchanovski, Address: Skopje 1000, North Macedonia. Email: bpopcan@msn.com
Cite as: Popchanovski, B, Balabanova-Stefanova, M, Dyslipidemia and hyperglycemia in psoriatic inpatients. Int J Med Students. 2019 Sep-Dec;7(4):62-5.
Copyright © 2019 Bojan Popchanovski, Margareta Balabanova-Stefanova
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 7, NUMBER 3, December 2019