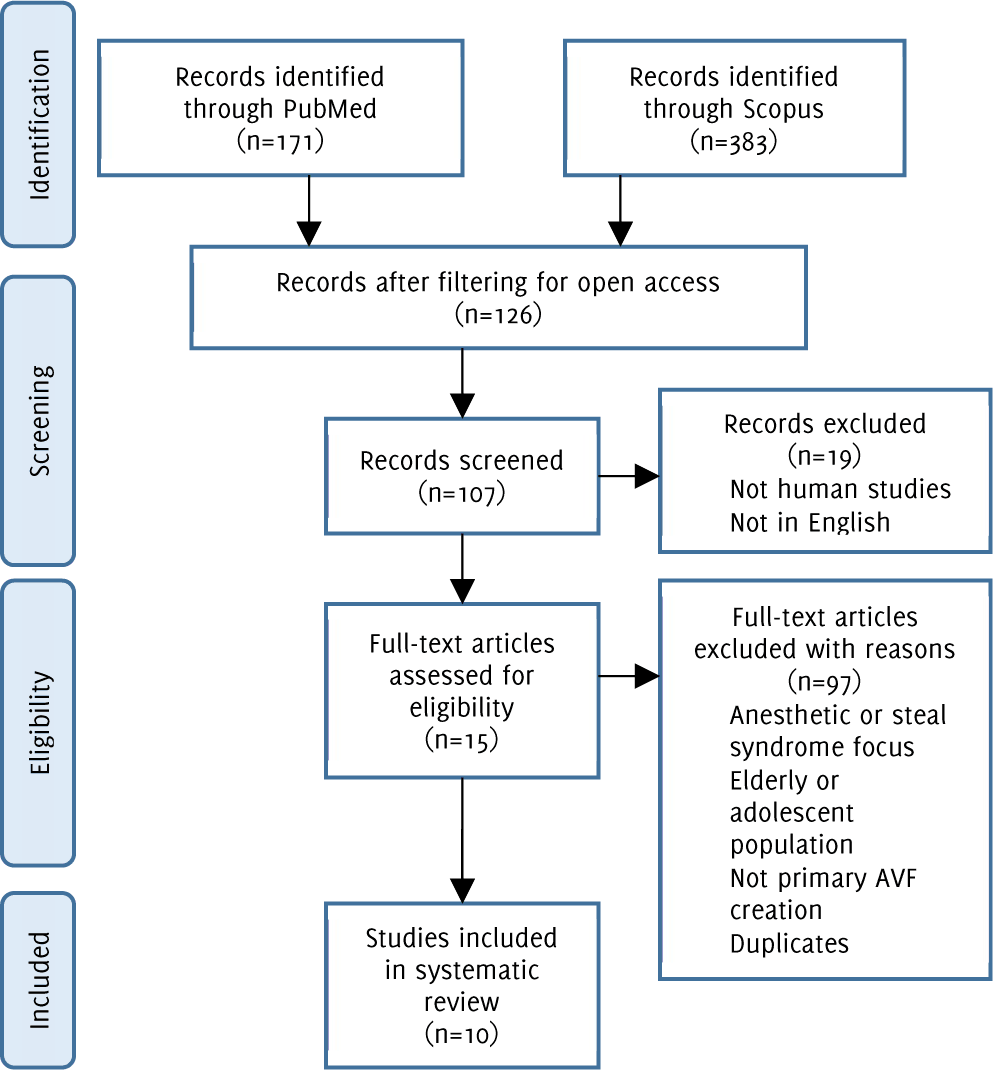
Flow diagram of study methodology with application of inclusion and exclusion criteria
Andrew Stanton Kucey1, Anish Engineer2, Shawn Stefan Albers1
doi: http://dx.doi.org/10.5195/ijms.2019.331
Volume 7, Number 3: 73-81
Received 31 10 2018: Rev-recd 23 11 2018: Rev-recd 22 02 2019: Accepted 06 10 2019
ABSTRACT
Chronic Kidney Disease (CKD) affects 10-16% of the US population and its incidence is rising due to increasing prevalence of associated risk factors. Renal replacement therapy is required to treat late stage CKD and hemodialysis is the preferred modality for many patients. Vascular access is required for hemodialysis and arteriovenous fistulas (AVF) are currently the gold standard. This review intended to collate current knowledge on AVF outcomes regarding both the patient and fistula. Scopus and Medline were utilized to identify relevant literature. Inclusion and exclusion criteria were applied to narrow search results. Among CKD patients, 33.5-77.4% require a central venous catheter (CVC) before dialysis through a fistula. Many patients (33-51%) use a CVC regardless of AVF creation due to fistula immaturity or failure. There are large variations in AVF creation policies internationally; 16% of American hemodialysis patients use a fistula compared to 72% of German patients. Primary patency and primary AVFs’ failure ranges from 60-70% and 20-26%, respectively. AVFs reduce morbidity and mortality in CKD. At present, too many patients are receiving hemodialysis through a CVC. Inadequate referral times for AVF creation can lead to fistula immaturity or failure in the intervention. Many countries are lagging behind recommended AVF creation rates published by the Kidney Disease Outcomes Quality Initiative. There is a paucity of literature concerning when a patient should be referred for AVF creation. It is paramount to have better predictive outcome measures and more clarity as to when patients will benefit from an AVF.
Keywords: Arteriovenous Fistula; Vascular Access Devices; Hemodialysis; Vascular Surgical Procedures; Chronic Renal Insufficiency (Source: MeSH-NLM).
Chronic Kidney Disease (CKD) is a pathologic condition resulting in a progressive decline of kidney function. It currently affects 7-12% of individuals globally, while its incidence is rapidly rising.1–4 The disease involves structural pathology such as nephron loss and fibrosis, which result in decreased glomerular filtration.5 These insults to the kidney contribute to systemic complications of CKD, including fluid and electrolyte abnormalities, anaemia, mineral-bone disorder, metabolic acidosis, hyperuricaemia, hypertension, dyslipidemia, cardiovascular disease and endocrine dysfunction.5 Severity of the disease can be divided into five stages depending on estimated glomerular filtration rate (eGFR), with substantial loss of kidney function and end-stage renal disease (ESRD) comprising stages IV and V, respectively.6–8 Renal replacement therapy is necessary once a patient has progressed to ESRD. Kidney transplants are ideal for renal replacement; however, they are not widely accessible. Consequently, hemodialysis (HD) is often the modality of choice for patients in ESRD.9
The process of HD clears the blood of uremic toxins using a series of pumps, membranes and dialysates. Patients undergoing this long-term therapy require permanent vascular access placement, as suggested by the Kidney Disease Outcomes Quality Initiative (KDOQI).1,8,10 The three main types of vascular access include arteriovenous fistulae (AVF), arteriovenous grafts (AVG) and central venous catheters (CVC).8 The current gold-standard for HD access is the formation of an AVF, as it is clinically reported to have better patient outcomes with reduced morbidity and improved survival.8,10–13 AVFs are established in the forearm through surgical anastomosis of a relatively small, peripheral artery with a larger subcutaneous vein.14 According to the KDOQI, the optimal timing for AVF creation is 6 months before cannulation, however this maturation can be affected by factors, such as age and gender.8,11 In the case where a patient's vascular integrity does not support a fistula, an AVG can be implemented. Studies indicate that AVGs have more drawbacks compared to AVFs. These include higher infection susceptibility potentially resulting in sepsis, reduced patency, and greater risk of complications ultimately leading to repeated interventions and diminished survival.15 CVCs, on the other hand, are generally employed prior to AVF or AVG maturation, when immediate initiation of HD is required. Infection and thrombosis among other life threatening complications, are persisting impediments.16 For instance, Lee et al. noted that catheter-related bacteremia was present in half of all HD patients studied 6 months after CVC implantation.17 Furthermore, HD patients with CVCs have a 3.43-fold increase in relative mortality risk compared to patients with an AVF.17–19
Therefore, AVFs are the gold standard method to attain vascular access in patients undergoing HD, as they result in fewer complications when compared to CVCs and AVGs.8,10–13 However, to better maintain access sites and retain the integrity of the vasculature, radiocephalic AVFs (RCAVF) are the recommended option by the KDOQI, and are associated with improved patient survival.12,20 In cases where the creation of a RCAVF is not feasible due to poor vasculature brachiocephalic, brachiobasilic, and brachiobrachial AVFs can be created.21 In spite of the clear benefits provided by AVFs for HD patients, they do carry some drawbacks and can potentially pose a risk for serious complications requiring hospitalization.22 The reported complications surrounding fistulas include aneurysm development, stenosis of the vein, dialysis-associated steal syndrome (due to ischemia), thrombosis, and infection.22 Additionally, the primary failure rates of AVF formation and maturation are approximated at 23% and 20-60%, respectively.1,23–25 Indeed, AVFs are a source of patient morbidity, however, they still remain the principal type of vascular access for HD compared to AVGs or CVCs.8,21 To ensure its success, the timing of AVF creation relative to HD must be considered. As there exists a long lag time between AVF formation and usage, fistulas can be created in a pre-emptive manner to circumvent potential CVC access, if HD is required. Even so, if the AVF is not needed or not used for access by the patient, the surgical procedure to create the fistula can result in unwarranted patient distress.1 Moreover, the average maturation time for an AVF falls between 148 to 285 days, with a 75% successful cannulation rate at 16 weeks (112 days) post-surgery.1,25,26
Taking the aforementioned factors into account, the temporally sensitive nature of this complex therapeutic intervention must be considered at the time of consultation. The time-course for formation of the fistula needs to be appropriately managed from initial patient referral to when the mature AVF will be needed. As the incidence of CKD rises, more patients will require renal replacement therapy and the creation of an AVF for HD. Therefore, it is imperative that we analyze and examine current practices to determine the best course of action.
This review will analyze the literature on arteriovenous fistulas used for vascular access to determine the current paradigms on:
An electronic search was conducted using the databases of Medline (PubMed) and Scopus. The search identified publications pertaining to the objectives and research question of the current study.
("arteriovenous fistula"[MeSH Terms]) OR ("arteriovenous"[All Fields] AND "fistula"[All Fields]) OR ("arteriovenous fistula"[All Fields] AND “creation”[All Fields] AND "blood vessels"[MeSH Terms]) OR ("blood"[All Fields] AND "vessels"[All Fields]) OR ("blood vessels"[All Fields]) OR ("vascular"[All Fields]) AND “access”[All Fields] AND “outcomes”[All Fields])
This search returned 171 results. The results were narrowed down to 61 publications after filtering for free, full-text, and again to 49 results after specifying for human studies. The inclusion and exclusion criteria were applied to the abstracts of the remaining 49 papers. This narrowed the search to 6 papers. The abstracts were screened by all three authors and conflicts regarding inclusion and exclusion were resolved through group meetings.
(arteriovenous AND fistula AND creation AND for AND vascular AND access AND outcomes)
This search returned 383 results. After filtering for open access publications, 65 papers remained. Filtering for human studies, English availability, articles, and reviews narrowed the results to 58. The remaining articles had their abstract subject to the inclusion and exclusion criteria. Nine publications were identified based on this, 5 of the papers overlapped with the Medline results.
In total, 10 articles were identified between the two database searches (Figure 1).
Figure 1.Flow diagram of study methodology with application of inclusion and exclusion criteria

The inclusion and exclusion criteria were generated a priori. Studies were included if they addressed populations who underwent AVF creation for HD access, if primary patency or AVF maturation were mentioned in the abstract and if the authors reviewed the outcomes of their subjects after AVF creation. The selected manuscripts all present new data published in its first report and are not review papers.
Exclusion criteria specified studies carried out on non-human species, articles not available in English, and articles that were not available as Open Access. Additionally, papers addressing exclusively elderly or adolescent populations were removed. Finally, articles were excluded if the study evaluated endovascular fistula creation, focused primarily on anaesthetic technique or on steal syndrome, or if the study addressed AVF interventions and revisions rather than primary creation, it was excluded.
All of the papers identified by the search methods were assessed for their quality and validity using the Evidence-based librarianship (EBL) critical appraisal tool.27,28 The abbreviated results are displayed in Table 1 and the extended appraisal can be viewed in Appendix A. All of the selected articles were deemed valid when applying the EBL criteria.27 The methodology of all the papers identified to be eligible for review were adequate and minimized bias that the individual study may be susceptible to.
Table 1Results of the EBL Critical Appraisal of selected articles for review.
| Article | Population Validity Score (%) | Data Collection Validity Score (%) | Design Validity Score (%) | Results Validity Score (%) | Overall Score (%) |
|---|---|---|---|---|---|
| Al-Jaishi et al (2015)29 | 100 | 80 | 100 | 100 | 95 |
| Biuckians et al (2008)26 | 75 | 80 | 100 | 100 | 90 |
| Dageforde et al (2015)30 | 75 | 80 | 100 | 100 | 90 |
| Ethier et al (2008)13 | 66 | 80 | 100 | 100 | 86 |
| Kimball et al (2011)1 | 75 | 80 | 100 | 100 | 90 |
| Korepta et al (2016)21 | 66 | 80 | 100 | 100 | 86 |
| Lee et al (2012)33 | 66 | 80 | 100 | 100 | 86 |
| Masengu et al (2016)32 | 75 | 80 | 100 | 100 | 90 |
| Schinstock et al (2001)31 | 100 | 80 | 100 | 100 | 95 |
| Wilmink et al (2016)25 | 100 | 80 | 100 | 100 | 95 |
A retrospective study looking at HD patients in Canada showed that 27% patients had at least one AV access created in a study population of 17,183 (Table 2).29 Of the patients who had an AVF created, 65% were able to cannulate it for HD, while 33% had to resort to a CVC.29 Of the patients who were referred late to a nephrologist, 8% had an AV access created as opposed to 39% who were referred early.29 Prior to AVF creation, CVC use occurred in 35.7-77.4% of patients.13,21,26,30,31 A study investigating the natural history of AVFs noted that 66% of vascular access procedures were to create an AVF, with 33% of these being RCAVFs.26 Four studies reported that 48-75% of AVFs were being used for HD at the time of follow-up, while 37-51% of them were abandoned.1,25,26,30,32 A study on pre-emptive AVF creation showed 49% of patients ended up on HD during their 10 month follow-up with 65% of these patients being dialyzed through their AVF.1 This paper also reported that 23% of the patients never used their viable AVF for HD.1
Table 2Summary of reviewed publications, arranged alphabetically.
| Authors and Location | Title | Objectives | Study Design | Sample Size | Population | Key Findings |
|---|---|---|---|---|---|---|
| Ahmed A. Al-Jaishi, Charmaine E. Lok, Amit X. Garg, Joyce C. Zhang, Louise M. Moist (2015). London, Canada.29 | Vascular access creation before hemodialysis initiation and use: a population-based cohort study | To assess how many patients had AV access before HD To elucidate secular trends in AV access To estimate the effect of referral time on AV access creation |
Retrospective population-based cohort study | n = 17,183 | The study population consisted of adults who used HD as their first modality for renal replacement therapy between January 1, 2001 and December 31, 2010 | 27% of patients had at least one AV access created with a median time of 184 days
between the procedure and HD commencement 65% of patients with an AV access were able to use it for dialysis, while 33% still had to use a CVC From 2001-2010 there was a decline in AV access creation before HD commencement; 32% to 22% (P < 0.001) 8% of patients with a late referral to nephrology had an AVG or AVF created, compared to 39% with an early referral |
| Andre Biuckians, Eric C. Scott, George H. Meier, Jean M. Panneton, Marc H. Glickman (2008). Norfolk, USA.26 | The natural history of autologous fistulas as first-time dialysis access in the KDOQI era | To determine the natural history of AVFs in first time vascular access patients | Retrospe ctive chart review | n = 80 | The study population consisted of all patients undergoing their first AVF creation from January 1, 2005 until June 30, 2005 in a single vascular practice | 75% of AV access candidates had prior CVC HD use 67% of first-time access patients had an AVF created, 33% had an AVG 33% received an RCAVF, 67% had a BCAVF. Time to first cannulation was significantly shorter in BCAVF than RCAVF (P=0.03) 48% of AVF were being used for HD at follow up (mean time of 278 days), and 11% matured without intervention Average maturation time was 148 days, cumulative functional patency at 1 year was 63% 48% of AVFs were being used for HD 37% of the AVFs were abandoned, 20% being from primary failure |
| Leigh Anne Dageforde, Kelly A. Harms, Irene D. Feurer, David Shaffer (2015). Nashville, USA.30 | Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency | To determine whether vein diameter measured by preoperative duplex ultrasound is associated with fistula maturation and secondary patency | Retrospe ctive chart review | n = 158 | The study population consisted of patients who had an AVF created from February 2009 until June 2011 | Larger minimum vein diameter was associated with lower risk for AVF failure Greater than 33% of veins < 2.7mm failed to mature at 6 months Multivariate analysis showed that for every 1mm increase in minimum vein diameter, the risk of failure to mature was reduced by 45% and there was a 36% reduction in risk of fistula failure (HR, 0.555; P=0.005 & HR, 0.639; P=0.001 respectively) 20% of fistulas failed to mature 53% of fistulas were used for HD within the study period 49% of patients had prior tunnelled catheter use for HD |
| Jean Ethier, David C. Mendelssohn, Stacey J. Elder, Takeshi Hasegawa, Tadao Akizawa, Takashi Akiba, Bernard J. Canaud, Ronald L. Pisoni (2008). Ann Arbor, USA.13 | Vascular access use and outcomes: an international perspective from the dialysis outcomes and practice patterns study | To describe changes in vascular access trends since the dialysis outcomes and practice patterns study (DOPPS) | Retrospe ctive review of a prospective database | DOPPS I n =16402 DOPPS II n =12839 DOPPS III n =7921 n = 37,162 |
The study population was HD patients at participating centres from: France, Germany, Italy, Japan, Spain, the USA and the UK in DOPPS I. Centres in Australia, Belgium, Canada, Sweden and New Zealand were added for DOPPS II and III. | In the interval between DOPPS I and DOPPS III, the use of AVFs in the USA increased
from 24% to 47%. AVF use also increased in the UK, Australia, and New Zealand Spain, Italy and Germany saw their AVF use decline AVG use declined in all countries, the largest fall was 58% to 29% in the USA CVC use increased in Europe, Canada and the USA from DOPPS I to III; 50% of patients from DOPPS II initiated HD with a CVC 35.7% of patients used a CVC if they had seen a nephrologist ≥ 4 months prior to HD start, as opposed to 77.4% who were seen < 1 month prior to HD Patients with longer wait times for surgical referral and evaluation had lower odds of initiating HD with an AVF (AOR 0.89 per 5 days longer; P<0.0001) |
| Traci A. Kimball, Ken Barz, Kelly R. Dimond, James M. Edwards, and Mark R. Nehler (2011). Denver, Colorado and Portland, Oregon, USA.1 | Efficiency of the kidney disease outcomes quality initiative guidelines for preemptive vascular access in an academic setting | To determine the efficiency of prophylactic AVF for HD To determine the incidence of HD within late stage CKD patients To determine AVF functional patency and morbidity within study population |
Retrospe ctive chart review | n = 150 | The study population was comprised of late stage CKD patients who underwent preemptive AVF creation across 2 academic centers. | Median time from surgical consultation to AVF creation was 31 days (min, max; 1, 400) At 10-month follow-up, 49% of patients were on HD and 65% of them were using their AVF 35% of patients on HD who were not using their AVF did not have AVF failure 23% never initiated HD but had a viable AVF, 28% never went on HD and also abandoned their AVF Mean maturation time for AVFs being used for HD was 285 days Incidence of AVF abandonment was 51% |
| Lindsey M. Korepta, Jennifer J. Watson, Erin A. Elder, Alan T. Davis, M. Ashraf Mansour, Christopher M. Chambers, Robert F. Cuff, Peter Y. Wong (2016). Grand Rapids, USA.21 | Outcomes for forearm and upper arm arteriovenous fistula creation with the transposition technique | To examine the rates of complication, failure, maturation, and patency in forearm cephalic vein (FACVT), upperarm cephalic vein (UACVT), and upper arm basilic vein transpositions (UABVT) for AVF creation | Retrospe ctive chart review | n = 165 | All patients undergoing AVF creation via FACVT, UACVT, or UABVT from January 1, 2006 until December 31, 2012. | 57% of patients were using a tunneled CVC for HD before AVF creation Average vein diameter was 3.2mm to 3.9mm No significant differences between groups in terms of time to maturation, primary 1-year patency (63-70%), or primary assisted 1-year patency (93-100%) 84% of FACVT, 88% of UACVT, and 86% of UABVT patients were able to use their AVF Average time to cannulation from when the AVF was created was 9.9±4.7 weeks |
| J.H. Lee, J.H. Won, C.K. Oh, H.A. Jung (2012). Suwon, Republic of Korea.33 | Clinical significance of upper-arm cephalic vein patency in autogenous radio-cephalic wrist fistulas for hemodialysis | To determine the role of upper-arm cephalic veins in RCAVF clinical outcomes | Retrospe ctive chart review | n = 183 | The study population included consecutive patients having an RCAVF created from March
2003-February 2009. Patients were divided into two groups depending on their upper-arm cephalic vein; stenosed or occluded were group B, group A had a patent lumen. |
Multivariate analysis showed upper arm cephalic vein status to be a predictor of primary
and secondary patency in RCAVFs (P<0.005) Maturation failure within 8 weeks was significantly higher in group B (26.7% vs. 9.8% in group A; P<0.009) Group A had significantly longer mean primary patency (P=0.011), however secondary patency was not significantly different between groups A and B Overall primary patency and secondary patency were both significantly greater in group A than B (P<0.0001) |
| Agnes Masengu, James McDaid, Alexander P. Maxwell, Jennifer B. Hanko (2016). Belfast, UK.32 | Preoperative radial artery volume flow is predictive of arteriovenous fistula outcomes | To determine whether pre-operative ultrasound analysis of vessels can predict AVF outcomes | Retrospe ctive cohort study | n = 152 | The study population consisted of all patients from a single left who underwent AVF creation after ultrasound mapping of their blood vessels (from August 2011-December 2014). | RCAVFs with arterial flow less than 50ml/min failed to mature 7 times more often than
those with higher flow rates (P<0.001) Radial artery volume flow < 50ml/min is a more sensitive measure for fistula failure to mature than mean vessel diameter of < 2.7mm 69% of the AVFs were functionally patent, 60% of the AVFs achieved primary patency 45% of AVFs failed to mature and were abandoned Females were associated with higher AVF failure to mature |
| Carrie A. Schinstock, Robert C. Albright, Amy W. Williams, John J. Dillon, Eric J. Bergstralh, Bernice M. Jenson, James T. McCarthy, Karl A. Nath (2011). Rochester, USA.31 | Outcomes of arteriovenous fistula creation after the fistula first initiative | To determine the outcomes of AVFs created at a single clinic and factors that predict their patency | Retrospe ctive cohort study | n = 293 | The study population was comprised of patients over 18 years old who were undergoing their first AVF creation the Mayo Clinic. | 50.5% of AVFs were created after HD commencement Kaplan-Meir survival at 3, 6, 12, and 18 months for primary patency was 67%, 50%, 41%, and 30%; secondary patency was 92%, 86%, 77%, and 73% respectively Univariate analysis showed arterial diameter predicts the primary and secondary AVF patency (HR, 0.83; 95% CI, 0.73 to 0.94 and HR, 0.67; 95% CI, 0.55 to 0.82 respectively) Multivariate analysis showed that diabetes increased the risk for lower primary patency (HR, 1.45; 95% CI, 1.06 to 1.99) and arterial diameter significantly influenced secondary patency (HR, 0.69; 95% CI, 0.56 to 0.84) Primary failure occurred in 26% of AVF 21.2% of AVF incurred a complication, and 54% of interventions happened before it was viable for HD |
| T. Wilmink, L. Hollingworth, S. Powers, C. Allen, I. Dasgupta (2016). Birmingham, UK.25 | Natural history of common autologous arteriovenous fistulae: consequences for planning of dialysis access | To determine primary failure, maturation times, and survival of common AVFs in order to aid future AVF planning | Retrospective longitudinal cohort study | n = 1,206 fistula operations | The study population consisted of patients undergoing AVF creation at a single centre from December 1, 2002-December 31, 2011. | Primary failure occurred in 23% of AVFs in the study; 75% were needled for dialysis
and 74% were functional RCAVFs had better survival than other AVFs, leading to more cumulative dialysis time Pre-dialysis AVF creation resulted in better AVF survival 10 weeks of maturation should be allowed before commencing dialysis on an AVF for 50% survival, 16 weeks for 75% survival Irrespective of age, the best available option is RCAVF creation 4 months before estimated dialysis commencement date |
A paper which assessed vascular access use since the dialysis outcomes and practice patterns study, found that 27% of Canadian and16% American HD patients were being dialyzed through an AVF.13 This is in contrast to 72% of German and 69% of Japanese HD patients who utilized an AVF.13 The proportion of HD patients using an AVG was highest in America at 15%, followed by Sweden at 9%.13
Four studies reported an average AVF maturation time of 148-285 days with a cumulative functional 1-year patency of 60-70%.1,21,26,32 Primary failure was recorded in 3 studies and occurred in 20-26% of cases.25,26,31 In one study, AVFs failed to mature 20% of the time.30 Primary assisted 1-year patency was only measured in one study, and it was found to be 93-100%.21 Complications occurred in 21.2% of AVFs and 54% of interventions occurred before maturation was achieved.31 RCAVF were shown to have a higher primary failure rate but better overall survival than brachiocephalic and brachiobasilic fistulas.25 When the AVFs were allowed to mature for 10 and 16 weeks, they had a 50% and 75% survival respectively upon cannulation.25
Dageforde et al. showed that minimum vein diameter is associated with lower risk for AVF failure.30 Veins < 2.7 mm in diameter had > 33% failure to mature at 6 months.30 A patent upper arm cephalic vein was shown to improve primary patency, secondary patency and maturation in patients undergoing RCAVF creation.33 RCAVFs with arterial flow rates < 50 mL/min were shown to have a 7 fold increase in failure rate.32 The flow rate was also shown to be a more sensitive marker than vein diameter when assessing failure to mature.32
Cox regression analysis associated female gender, being on dialysis at the time of AVF creation, and diabetes with worse AVF survival.25 Dageforde et al. also showed that preoperative dialysis was associated with higher risk of AVF failure.30 The study by Wilmink et al. also demonstrated that females were associated with higher primary failure and longer maturation times.25 One study found that an age ≥ 65 years was an independent predictor of secondary AVF patency.33 Those less likely to have an AVF created were females, as well as patients with a high number of comorbidities.29 Gender was shown to be unassociated with primary or secondary patency by Schinstock et al., however, body mass index (BMI), diabetes, AVF site, previous CVC use, and the diameter of the artery, were all associated with primary patency.31 While diabetes did not have an association with secondary patency, increased age and thromboembolic disease status were related to secondary patency in addition to the aforementioned factors.31 Kimball et al. found no relation between sex, BMI, smoking, age, race, fistula location and rate of AVF abandonment.1 Another study failed to identify individual predictors of AVF failure from factors including smoking, age, sex, BMI, diabetes, hypertension and hyperlipidemia.21 Finally, in a study looking at minimum vein diameter for AVF outcome prediction, coronary artery disease was associated with a lower risk of AVF failure overall.30
This systematic review intends to combine recent investigations on outcomes of patients who are referred for AVF creation. The outcomes were subdivided into those that pertained to patient prognosis and those that measured the success of the AVF itself. The findings of this review suggest that patients are not being referred at an adequate time for AVF creation based on current KDOQI guidelines as 33.5-77.4% of patients are requiring a CVC use before they are using their AVF for HD.8 Even once an AVF was made, 33-51% of the patients still ended up on a CVC for HD due to AVF immaturity or failure.1,25,26,29,30,32
There were vast differences between countries in regards to the uptake of the KDOQI recommendations for AVF implementation. These guidelines suggest that 65% of HD patients should be using a fistula for their HD sessions.8,29 Only 27% and 16% of HD patients from Canada and USA, respectively, were being dialyzed through an AVF.13 In contrast, 72% of German and 69% of Japanese HD patients were using an AVF.13 These stark contrasts between nations may reflect local policies regarding healthcare or surgical preference, as 15% of American HD patients were using AVGs, while the next highest country was Sweden with 9%.13 The level of access to vascular surgeons may also impact results across regions. This is especially applicable to the Canadian and American healthcare systems which have some of the longest wait times between AVF consultation and AVF creation.13,34 Encouragingly, the use of AVFs is on the rise in most countries while the use of AVGs is on the decline13 Between 1996 and 2007, the largest changes occurred in the US where the use of AVFs jumped from 24% to 47%, while AVG usage dropped from 58% to 29% in HD patients.13 Despite the numerous drawbacks of an AVF procedure, it is still the best option of the available AV access modalities, and the literature supports its utility in terms of patient prognosis over AVGs and CVC.8,35
Regarding the fistulas themselves, the results of this study are consistent with what was reported in a meta-analysis by Al-Jaishi et al., in 2014.24 They reported a primary patency of 60% at 1 year and 51% at 2 years. Secondary patency was 71% and 64% at one and two years, respectively.24 The pooled primary failure from the same study was 23%.24 The current study found primary patency to be 60-70% while primary failure ranged from 20-26%. Secondary patency was difficult to estimate due to variations in reporting between the different publications. However, the abandonment rate for the fistulas ranged from 37-51%, which leads to the conclusion that the secondary patency may be lower in this review.
The success of AVFs can be predicted by multiple factors but the most accurate methods reviewed in this study are preoperative arterial flow rate and minimum vein diameter measurements.30,32 Both of these parameters had high predictive capability compared to factors such as sex, age, morbidity status, lifestyle factor and fistula site.30,32 Other than arterial flow rate and vein diameter, there was discordance between the publications as to whether other factors correlated with AVF outcomes or not.
All publications included in this review were deemed valid using the EBL critical appraisal tool. Using this method, a threshold of ≥ 75% of the specified criteria was necessary for validity in each individual section and cumulatively, in the individual articles. The lowest overall score was 86%, which was calculated for 3 different studies.13,21,33 The main area of methodological concern arose from population validity. Three studies were found to have assessed a poorly representative population, as all of them attained a score of 66%. Problematic areas for the population validity were: a small sample size, lack of clearly defined exclusion and inclusion criteria, and no randomization of subjects in comparative studies. For the rest of the validity calculation, all studies were found to be diligently designed other than the pervasive theme of ambiguity regarding whether the investigators played a role in delivering a service to the target population or not.
The articles included in this study were stringently examined using a standardized appraisal tool, reducing bias in calculating the validity of the selected publications. The limitations of this study are that there were only two databases used to identify publications to be included in the review. The database search was only carried out by one investigator which leaves the possibility for selection and reporting bias. This review is limited by an English availability filter used in the database search. The free-full text filter may have removed potentially relevant articles.
Future studies should be directed to the application of preoperative vasculature assessment for prediction of AVF outcomes. Further investigation into the reasons for late referrals to nephrology and vascular surgery in ESRD patients would also be beneficial. Finally, examining the rate of eGFR decline in patients to try and make standardized recommendations for when to refer them for AVF consultation based on their diminishing renal function could provide important and topical data.
The gold standard for vascular access is still the AVF. With the aging global population, there will be an increasing demand for dialysis, which necessitates better standardization regarding patient referral for AVF creation. There are large variations in vascular access use between countries, despite HD patients faring much better when being dialyzed through an AVF as opposed to AVGs or CVCs. A concerted effort is required to try and meet the KDOQI guidelines for timely vascular access creation, improved AVF function and enhanced patient survival.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: ASK. Methodology: ASK, AE, and SA. Formal Analysis: ASK. Investigation: ASK, AE, and SA. Writing – Original Draft: ASK. Writing – Review & Editing: ASK, AE, and SA. Visualization: ASK, AE, and SA. Supervision: ASK. Project Administration: ASK.
Appendix A Full EBL Critical Appraisal Checklist for all articles included in review.| EBL | Critical Appraisal Checklist27 | Al-Jaishi et al(2015)29 | Biuckians et al(2008)26 | Dageforde et al (2015)30 | Ethier et al (2008)13 | Kimball et al (2011)1 | Korepta et al (2016)21 | Lee et al (2012)33 | Masengu et al (2016)32 | Schinstock et al (2001)31 | Wilmink et al(2016)25 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Section A: Population | Is the study population representative of all users, actual and eligible, who might be included in the study? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Are inclusion and exclusion criteria definitively outlined? | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | |
| Is the sample size large enough for sufficiently precise estimates? | Y | N | Y | Y | N | N | N | N | Y | Y | |
| Is the response rate large enough for sufficiently precise estimates? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Is the choice of population bias-free? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| If a comparative study: a Were participants randomizedinto groups? b) Were the groups comparable atbaseline? c) If groups were not comparableat baseline, was incomparabilityaddressed by the authors in theanalysis? |
N/A | N/A | N/A | N Y N/A |
N/A | N Y N/A |
N Y N/A |
N/A | N/A | N/A | |
| Was informed consent obtained? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Section A validity: | 100% | 75% | 75% | 66% | 75% | 66% | 66% | 75% | 100% | 100% | |
| Section B: Data Collection | Are data collection methods clearly described? | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| If a face-to-face survey, were inter-observer and intra-observer bias reduced? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Is the data collection instrument validated? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| If based on regularly collected statistics, are the statistics free from subjectivity? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Does the study measure the outcome at a time appropriate for capturing the intervention's effect? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is the instrument included in the publication? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Are questions posed clearly enough to be able to elicit precise answers? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Were those involved in data collection not involved in delivering a service to the target population? | U | U | U | Y | U | U | U | U | U | U | |
| Section B validity: | 80% | 80% | 80% | 80% | 80% | 80% | 80% | 80% | 80% | 80% | |
| Section C: Design | Is the study type / methodology utilized appropriate? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is there face validity? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is the research methodology clearly stated at a level of detail that would allow its replication? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Was ethics approval obtained? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Are the outcomes clearly stated and discussed in relation to the data collection? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Section C validity: | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| Section D: Results | Are all the results clearly outlined? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are confounding variables accounted for? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Do the conclusions accurately reflect the analysis? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is subset analysis a minor, rather than a major, focus of the article? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Are suggestions provided for further areas to research? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is there external validity? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Section D validity: | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| Overall Validity Score: | 95% | 90% | 90% | 86% | 90% | 86% | 86% | 90% | 95% | 95% | |
1.Kimball TA, Barz K, Dimond KR, Edwards JM, Nehler MR. Efficiency of the kidney disease outcomes quality initiative guidelines for preemptive vascular access in an academic setting. J Vasc Surg. 2011 Sep;54 (3):760–5; discussion 765-6.
2.Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, Morrison H. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013Jun11;185 (9):E417–23
3.Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS One. 2016Jul6;11 (7):e0158765.
4.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P. Prevalence of chronic kidney disease in the United States. JAMA. 2007Nov7;298 (17):2038–47.
5.Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M. Chronic kidney disease. Nat Rev Dis Primers. 2017Nov23;3:17088.
6.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012Jan14;379 (9811):165–80.
7.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 suppl 1):S1–266.
8.Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Vol. 48 suppl 1, Am J Kidney Dis. 2006 Jul;48 suppl 1:S176–247.
9.Sinnakirouchenan R, Holley JL. Peritoneal dialysis versus hemodialysis: Risks, benefits, and access issues. Adv Chronic Kidney Dis. 2011 Nov;18 (6):428–32.
10.Perl J, Nessim SJ, Moist LM, Wald R, Na Y, Tennankore KK. Vascular Access Type and Patient and Technique Survival in Home Hemodialysis Patients: The Canadian Organ Replacement Register. Am J Kidney Dis. 2016 Feb;67 (2):251–9.
11.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ. Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol. 2013 Feb;24 (3):465–73.
12.III. NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: update 2000. Am J Kidney Dis. 2001 Jan;37(1 suppl 1):S137–81.
13.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008 Oct;23 (10):3219–26. .
14.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D. EBPG on vascular access. 1. Patient referral. European best practice guidelines on haemodialysis. Nephrol Dial Transplant. 2007May1;22:ii88–ii117
15.Leake AE, Yuo TH, Wu T, Fish L, Dillavou ED, Chaer RA. Arteriovenous grafts are associated with earlier catheter removal and fewer catheter days in the United States Renal Data System population. J Vasc Surg. 2015 Jul;62 (1):123–7.
16.Xue H, Ix JH, Wang W, Brunelli SM, Lazarus M, Hakim R. Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am J Kidney Dis. 2013 Jan;61 (1):123–30.
17.Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005 Sep;46 (3):501–8.
18.Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015 May;87 (5):996–1008.
19.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006 Mar;47 (3):469–77.
20.Leur K de, Öztürk Ç, Zeeland MLPV, Groot HGW de, Heyligers JMM, Vriens PWHE. Vascular Access Outcome in the Elderly Dialysis Patient in Combination With the Quality of Life. Vasc Endovascular Surg. 2013Aug6;47 (6):444–8.
21.Korepta LM, Watson JJ, Elder EA, Davis AT, Mansour MA, Chambers CM. Outcomes for forearm and upper arm arteriovenous fistula creation with the transposition technique. J Vasc Surg. 2016 Mar;63 (3):764–71.
22.Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22(3):220–8.
23.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis. A randomized controlled trial. JAMA. 2008May14;299 (18):2164–71.
24.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX. Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis. 2014 Mar;63 (3):464–78.
25.Wilmink T, Hollingworth L, Powers S, Allen C, Dasgupta I. Natural History of Common Autologous Arteriovenous Fistulae: Consequences for Planning of Dialysis Access. Eur J Vasc Endovasc Surg. 2016 Jan;51 (1):134–40.
26.Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg. 2008 Feb;47 (2):415–21; discussion 420-1.
27.Glynn L. EBL Critical Appraisal Checklist. Libr Hi Tech. 2006;24 (3):387–99.
28.Glynn L. A critical appraisal tool for library and information research. Library Hi Tech. Emerald Group Publishing Limited; 2006;24 (3):387–99.
29.Al-Jaishi AA, Lok CE, Garg AX, Zhang JC, Moist LM. Vascular access creation before hemodialysis initiation and use: a population-based cohort study. Clin J Am Soc Nephrol. 2015Mar6;10 (3):418–27.
30.Dageforde LA, Harms KA, Feurer ID, Shaffer D. Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency. J Vasc Surg. 2015 Jan;61 (1):170–6.
31.Schinstock CA, Albright RC, Williams AW, Dillon JJ, Bergstralh EJ, Jenson BM. Outcomes of Arteriovenous Fistula Creation after the Fistula First Initiative. Clin J Am Soc Nephrol. 2011 Aug;6 (8):1996–2002.
32.Masengu A, McDaid J, Maxwell AP, Hanko JB. Preoperative radial artery volume flow is predictive of arteriovenous fistula outcomes. J Vasc Surg. 2016 Feb;63 (2):429–35.
33.Lee JH, Won JH, Oh CK, Jung HA. Clinical significance of upper-arm cephalic vein patency in autogenous radial-cephalic wrist fistulas for hemodialysis. Eur J Vasc Endovasc Surg. 2012 Nov;44 (5):514–20.
34.Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL. Haemodialysis vascular access problems in Canada: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant. 2006 Mar;21 (3):721–8.
35.Young EW, Dykstra DM, Goodkin DA, Mapes DL, Wolfe RA, Held PJ. Hemodialysis vascular access preferences and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int. 2002 Jun;61 (6):2266–71.
Andrew Stanton Kucey, 1 BSc, MSc, Medical student, School of Medicine University College Cork, Ireland
Anish Engineer, 2 BMSc, PhD, School of Medicine, Royal College of Surgeons Ireland, Ireland
Shawn Stefan Albers, 1 BSc, MSc, Medical student, School of Medicine University College Cork, Ireland
David Avelar Rodriguez, Editor
Mihnea-Alexandru Găman, Editor
Madeleine Jemima Cox, Student Editor
About the Author: Andrew is currently a final year medical student at University College Cork, Ireland (4/4 years). He has attained an academic standard of first-class honors throughout his studies.
Correspondence: Andrew Stanton Kucey, Address: [Institution of affiliation address] Email: 116100312@umail.ucc.ie
Cite as: Kucey AS, Engineer A, Albers SS. Outcomes of Patients Referred for Arteriovenous Fistula Construction: A Systematic Review. Int J Med Students. 2019 Sep-Dec;7(3):73-81.
Copyright © 2019 Andrew Stanton Kucey, Anish Engineer, Shawn Stefan Albers
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 7, NUMBER 3, December 2019