Study Identification PRISMA Flow Chart; Template Adapted from Page et al. 8
Seon Hayles1, Kelsey Williams1, Nidhi Thomas1, Jabari Morgan1, Donna Braham2, Maxine Gossell-Williams3, Jonathan D. Ho4
doi: http://dx.doi.org/10.5195/ijms.2023.1648
Volume 11, Number 3: 220-228
Received 09 08 2022; Rev-request 24 09 2022; Rev-request 04 04 2023; Rev-recd 25 09 2022; Rev-recd 13 04 2023; Accepted 24 06 2023
ABSTRACT
Background:Pseudo-chilblains have been associated with COVID-19. Many reports, however, lack confirmation of COVID-19 infection. While likely associated, all chilblains/chilblain-like lesions during this time should not be assumed to be COVID-19 related. This study examines the characteristics of adults with pseudo-chilblains and confirmed COVID-19.
Methods:A systematic review of PubMed/MEDLINE database was performed using the PRISMA guidelines. Adults (>18 years) with confirmed COVID-19 were included. De-identified registries were excluded to avoid duplication. We extracted study design, age, sex, race, geographic location, relationship of COVID-19 diagnosis to chilblains onset, confirmatory testing, hospitalization status, anatomical location, cold/damp exposure, presence/absence/description of pseudo-chilblains symptoms, presence/absence of biopsies/histopathologic findings, tissue IHC/PCR, presence/absence/details of extracutaneous COVID-19 disease, pre-existing chilblains, treatment and resolution timeline. The search was completed in July 2022.
Results:We identified 13 studies (29 patients). In COVID-19-infected adults, pseudo-chilblains were reported primarily from North America and Europe, occurring in both sexes over a wide age-range, affected well and ill patients, favored the hands and feet and could be symptomatic or asymptomatic. Most patients had extracutaneous symptoms. Resolution time ranged from <1 week to >50 days. There was marked variation in treatment strategies and appearance of pseudo-chilblains relative to entire disease course. Biopsies were infrequently performed but findings similar to classical chilblains were described.
Conclusions:Many patients reported as pseudo-chilblains of COVID-19 lack confirmed infection. Infection confirmation, photographic documentation and histopathology are critical to establish homogeneity in reported pseudo-chilblains during this global pandemic. Further work clarifying the relationship of acral eruptions and COVID-19 is necessary.
Keywords: COVID-19; SARS CoV-2; Pernio; Perniosis; Chilblain; Exanthem; Viral; Toes; Pseudo-Chilblains; Severe Acute Respiratory Syndrome Coronavirus 2; Equelae; Post-acute sequelae SARS-CoV-2; Sequelae; Complications; Viral pneumonia; 2019-nCoV Infection; SARS-CoV-2 Infection; Coronavirus Disease 2019 (Source: MeSH-NLM).
Recent reports document cutaneous manifestations of coronavirus disease of 2019 (COVID-19) infection including exanthematous, urticarial, papulovesicular and vascular-related eruptions.1 Acral lesions described early in the pandemic were designated ‘pseudo-chilblains', ‘COVID-toes' or ‘chilblain-like' due to their resemblance to classical chilblains. Compared with classical chilblains, these patients lacked cold exposure but reported COVID-19 infection/exposure.1–3 The diagnosis has typically been made clinically in patients with erythematous to violaceous papules, plaques or occasionally blisters in confirmed or clinically suspicious cases of COVID-19 or in patients with compatible lesions and a recent exposure to known COVID-19 infection.1–5 The lesions may be painful, pruritic or asymptomatic and occur in both children and adults, with equal distribution between sexes. While the pathophysiology of pseudo-chilblains is still unclear, viral infection associated increased interferon-α, a strong cytotoxic T-cell and natural killer cell response, along with IgA anti-neutrophil cytoplasmic antibodies have been described.6 This immune response likely contributes to the dense perivascular and periadnexal lymphocytic infiltrate seen on histopathologic sections.6 Cryofibrinogenemia with potential resultant vascular microthrombi has also been reported as a potential pathomechanism.7 In addition to being a marker of COVD-19 positivity, prognostic implications have been suggested,4 with pseudo-chilblains reportedly associating with mild disease.4 One challenge with the data regarding its association with COVID-19 is the lack of confirmed infection in many studies and whether this eruption is a true manifestation of COVID-19 infection remains controversial.8 In many reports, infection was inferentially deduced using known contact exposure or previous suggestive clinical symptoms rather than confirmed laboratory testing.5 Although little doubt exists that pseudo-chilblains are a manifestation in some patients with COVID-19 infection, it should not be assumed that it is exclusively seen in COVID-19 infected patients.9 Lack of clinical criteria, variation in appearance and infrequently performed biopsies raise the possibility that pseudo-chilblains may not be a homogenous condition, potentially representing a variety of livid-appearing eruptions with differing pathomechanisms or prognostic implications. Thus, our study aims to describe the demographic, clinical and laboratory features of adult patients with pseudo-chilblains and confirmed COVID-19 infection.
A systematic review search strategy was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A literature search was done on July 14, 2022 and July 17, 2020 using PubMed/MEDLINE and Web of Sciences databases respectively. Following PRIMSA 2015.10 which requires at least two databases, we used those detailed above. We restricted data to scientific peer reviewed journals. We did not include gray literature. Gray literature is not formally peer reviewed work and thus did not meet our inclusion criteria. Many would also not have COVID-19 diagnostically confirmed. Our included keywords with Boolean terms were “Chilblains” OR “COVID toes” AND “COVID-19”, as well as “COVID-19” AND “Chilblains” AND “immunohistochemistry”. The search was filtered to only include journal articles, human adult studies (>18 years), written in English and published between January 2020 and June 30 2022. An additional search on Web of Science using the same Boolean terms was completed on July 17, 2022. Archiving of the review protocol was not previously done.
Two authors (SH, MG) independently screened titles/abstracts identifying and including articles describing pseudo-chilblains in patients with confirmed COVID-19 infection (defined as positive reverse transcriptase polymerase chain reaction (RT-PCR), positive serology for IgG/IgM or detection of COVID-19 on biopsies via immunohistochemistry/immunofluorescence (IHC/IF), in situ hybridization (ISH) or tissue PCR. Where there was disagreement on inclusion/exclusion a third author (KW) was consulted for consensus. Eligibility of study based on data available for extraction was determined through full-text review with consensus between two authors (SH, KW, NT, JM) and final review by consultant dermatologist (JH). Studies involving data extracted from de-identified patient registries, such as the American Academy of Dermatology Association COVID-19 Dermatology Registry (https://www.aad.org/member/practice/coronavirus/registry) were excluded to avoid duplicated patient representation. The inclusion/exclusion criteria were decided and vetted using multiple practice runs during planning meetings prior to July 14th. With the criteria decided, a single run was completed on July 14th, 2022 for PubMed and July 17th, 2022 for Web of Science. Microsoft Word was used to organize and manage the yielded citations. Once there was consensus on the included studies, Microsoft Excel was used to extract the required data from the papers.
Data extracted included study design, number of patients with confirmed COVD-19 and pseudo-chilblains, age, sex, race, geographic location, temporal relationship of COVID-19 diagnosis to onset of chilblains, confirmatory test used, hospitalization status, anatomical location, exposure to cold/damp, presence/absence and description of pseudo-chilblains related symptoms, presence/absence of a biopsy and where reported, histopathologic findings, tissue IHC/PCR, the presence/absence and details of extracutaneous COVID-19 disease, history of conventional chilblains, treatment and resolution timeline.
The Joanna Briggs Institute critical appraisal checklists (2017) for case reports, case series, cross-sectional and cohort studies11 were utilized to assess the overall quality of the included studies and estimate the risk for bias. For example, we assigned “Yes” to the question “Was the patient's history clearly described and presented as a timeline?” only if there was well-detailed chronology and timing of events reported. Similarly “Yes” would be assigned to “Were valid methods used for identification of the condition for all participants included in the case series?” only if a standard method of diagnosis was utilized (PCR, antibody testing etc.). All of our case reports and series had at minimum “Yes” assigned to criteria 1–4 and for cohort and cross-sectional studies, at minimum “Yes” assigned to criteria 1–3 and 7.
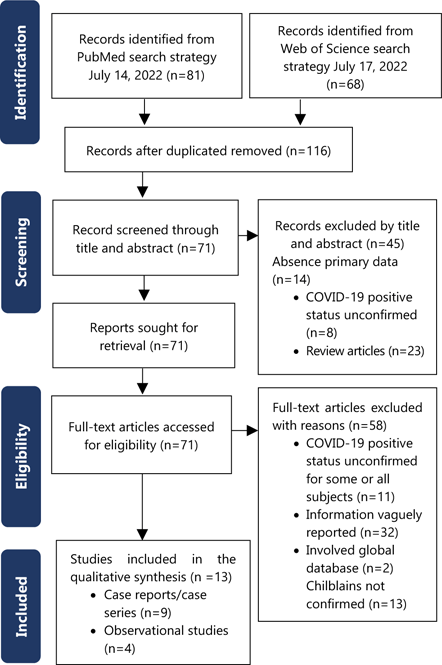
The flow diagram of the search and study selection process is shown in Figure 1. The literature search resulted in 116 articles which were evaluated for relevancy based on their titles and abstracts. Following title and abstract review, 45 studies were excluded for lack of confirmed infection (n=8) or absence of primary data (n=14). Review articles were also excluded (n=23). Seventy-one articles remained for full text reading. Of these, 58 were excluded for lack of confirmed infection in some/all subjects (n=11), inability to extract data due to vague reporting (n=32), lack of confirmed clinical features of chilblains-like lesions (n=13) and global databases (n=2). The subsequent review of full texts yielded 13 articles which fulfilled the selection criteria to be included in the systematic analysis. 13–25 Extracted data is shown in Table 1 and Table 2. There were four observational studies and nine case reports/case series. As it relates to confirmation of COVID-19 infection, five studies used both nasopharyngeal RT-PCR and serologic IgM/IgG testing for COVID-19, four with RT-PCR only, one study solely through serologic antibody testing, two via positive spike protein IHC/IF on biopsies and one study used all three methods.
Study Identification PRISMA Flow Chart; Template Adapted from Page et al. 8

Clinical/Laboratory Characteristics of Chilblain-like Lesions in Adults with Confirmed COVID-19 Infection (Part A).
| Authors | Country (C) Ethnicity (E) | Study Design & Number of cases (n) | Sex (M: F) & Age (years)* | Type of COVID-19 confirmatory test | Hospitalization status | Pseudo-chilblains presentation relative to overall course of COVID-19 infection |
|---|---|---|---|---|---|---|
| Almeida et al. (2021)14 | C: Brazil & USA E: NR |
Case Series n=4 |
4M 25,49, 62,66 |
RT-PCR/antibody serology | Outpatient | NR |
| Alramthan and Aldaraji (2020)21 | C: Qatar E: NR |
Case report n=2 |
2F 27,35 |
RT-PCR | Outpatient | NR |
| Brancaccio et al. (2021) 22 | C: Italy E: NR |
Cross-sectional n=2 |
1M:1F 19,29 |
IgG/IgM serology (RT-PCR negative) | Outpatient | Days 3 and 13 after onset of COVID-19 symptoms |
| Gambichler et al. (2020) 23 | C: Germany E: NR |
Case report n=1 |
1F 80 |
RT-PCR/IgG antibody serology/IHC | Inpatient | 3 weeks |
| Ko et al. (2021)25 | C: USA E: NR |
Case series n=3 |
1M:2F 82,62,76 |
IHC tissue | NR | NR |
| Mendez-Maestro et al. (2020)18 | C: Spain E: NR |
Cross-sectional n=6 |
NR 64–70 |
RT-PCR/antibody serology | Inpatient | NR |
| Proietti et al. (2020) 24 | C: Italy E: White |
Case report n=1 |
F 35 |
RT-PCR | Outpatient | 14 days after positive PCR |
| Recalcati et al. (2021)16 | C: Italy E: NR |
Observational Retrospective cohort n=2 |
2F 31, 33 |
RT-PCR (n=1), ELISA (n=1) | Outpatient | 2 weeks after extracutaneous COVID-19 symptoms (n=1) First day of presentation (n=1) |
| Rekhtman et al. (2021)17 | C: USA E: White, Black, Asian, Native American, Hispanic, Multiracial (not specifically stated for each case) |
Observational Prospective cohort n=4 |
NR 55–77 |
RT-PCR/antibody serology | Inpatient | NR |
| Rubin et al. (2020)15 | C: USA E: NR |
Case report n=1 |
1F 27 |
RT-PCR | Outpatient | 6 weeks after extracu-taneous symptoms |
| Santonja et al. (2020)13 | C: Spain E: NR |
Case report n=1 |
1F 36 |
IHC tissue (RT-PCR + IgG/IgM serology negative) | Outpatient | First day of presentation |
| Shah et al. (2021)20 | C: USA E: NR |
Case report n=1 |
1M 19 |
Antibody serology | Outpatient | First day of presentation |
| Wee and Tey (2020)19 | C: Singapore E: Asian (Indian) |
Case report n=1 |
1M 26 |
RT-PCR | Outpatient | NR |
Legend:
*Where specific ages not available, age-range of cohort reported; IHC, Immunohistochemistry; NR, Not reported; RT-PCR, reverse transcriptase polymerase change reaction.Clinical/Laboratory Characteristics of Chilblain-like Lesions in Adults with Confirmed COVID-19 Infection (Part B).
| Authors | Extracutaneous COVID-19 symptoms/cases number | Cold/damp exposure | Anatomical location(s)** | Symptoms related to pseudo-chilblains | Histopathologic findings | Pseudo-chilblains specific treatment | Time to resolution |
|---|---|---|---|---|---|---|---|
| Almeida ei al. (2021)14 | Fever, headache and diarrhea (1/4 cases) Asymptomatic (3/4 cases) |
NR | Toes (n=4) Fingers (n=1) Ears (n=1) |
Pruritus (n=1) Asymptomatic (n=3) |
-Spongiotic dermatitis with vesicles -Keratinocyte necrosis (dyshidrotic pattern) -Superficial perivascular lymphocyte infiltrate |
NR | Day: 7, 11, 12, 15 days |
| Alramthan and Aldaraji (2020)21 | Asymptomatic (2/2 cases) | NR | Fingers on bilateral hands (n=2) | Asymptomatic (n=2) | Not performed | NR | NR |
| Brancaccio et al. (2021)22 | Mild symptoms not otherwise described (2/2 cases) | NR | Toes and fingers (n=1) Toes (n=1) |
Pain (n=2) | Not performed | None (2/2 cases) | Day: 14, 7 |
| Gambichler et al. (2021)23 | Fever, cough shortness of breath, COVID pneurmonia (1/1 cases) | NR | Thumb (n=1) | Asymptomatic (n=1) | -Parakeratosis, acanthosis -Perivascular and diffuse lymphohistiocytic infiltrate -Fibrinoid deposits and occlusion of mid-dermal blood vessels -IF positive for SARS-CoV-2 spike protein |
None | NR |
| Ko et al. (2021)25 | NR (3/3 cases) | NR | Fingers and toes (individual case details not specified) | NR | Perivascular lymphocytic infiltrate IHC: + spike protein |
NR | NR |
| Mendez-Maestro et al. (2020)18 | NR (6/6 cases) | Unrelated to exposure | Toes and fingers (individual case details not specified) | Asymptomatic (n=6) | Not performed | Observation (6/6 cases) | Resolved, but timeline not reported |
| Proietti et al. (2020) 24 | Asymptomatic (1/1 cases) | NR | Right auricle | Pain | Not performed | Methylprednisolone Heparin (1/1 cases) |
5 |
| Recalcati et al. (2021)16 | Fever (1/2 cases) Asymptomatic (1/2 cases) |
Unrelated to exposure | Hands (n=1) Feet (n=2) |
Asymptomatic (n=2) | -Dense coat-sleeve-like perivascular and perieccrine lymphocytic infiltrate | Observation (2/2 cases) | Day: 20, 21 |
| Rekhtman et al. (2021)17 | NR (4/4 cases) | NR | Hand (n=1) Fingers (n=3) Feet (n=1) Toes (n=2) |
NR | Not performed | NR | NR |
| Rubin et al. (2020)15 | Anosmia, Ageusia (1/1 cases) | Unrelated to exposure | Toes | Swelling, pruritus | None performed | Observation | 3 months |
| Santonja et al. (2020)13 | Fever, cough (1/1 cases) | NR | Toes | NR | -Perivascular and periadnexal lymphocytic infiltrate -Focal thrombosis -Focal endothelial damage -DIF: perivascular C3 C1q and C5b-9 -IHC: + spike protein |
LMW heparin Aspirin | Day 54 |
| Shah et al. (2021)20 | Asymptomatic (1/1 cases) | Unrelated to exposure | Toes | Pain, blisters, tightness | Not performed | NSAID | Day 40 (faint cyanosis remained) |
| Wee and Tey (2020)19 | Asymptomatic (1/1 cases) | NR | Left thumb and palm (n=1) | Pain, swelling | Not performed | Paracetamol | Day 12 (palm) |
Legend:
**An individual case may have more than one anatomic location involved; IF, Direct immunofluorescence; IHC, Immunohistochemistry; IF, immunofluorescence; LMW, low molecular weight; NR, Not reported; NSAID, Non-steroidal anti-inflammatory drug.The majority of included studies fulfilled most of the study-type appropriate Joanna Briggs Institute Critical Assessment checklist parameters (Tables 3–4). For case reports/series missing information was primarily related to the adverse reactions which were generally not relevant based on the subject being studied. Similarly, for observational studies (cohort and cross-sectional studies), information on confounders was not generally available. Overall, based on the assessment of the critical appraisal checklists, all but one of our studies had >70% “yes” answers to relevant/applicable criteria (See Table 3–4). Therefore, while not negligible, we assessed the risk of bias as relatively low.
Results of Joanna Briggs Institute Critical Appraisal Checklists for Case reports and Case Series.
| Study Type (CS/CR), Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 |
|---|---|---|---|---|---|---|---|---|---|---|
| CS, Almeida et al. (2021)14 | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A |
| CR, Alramthan and Aldaraji, (2020) 21 | Y | Y | Y | Y | N | N | N/A | Y | - | - |
| CR, Gambichler et al. (2020) 23 | Y | Y | Y | Y | Y | Y | N/A | Y | - | - |
| CS, Ko et al. (2021)25 | Y | Y | Y | Y | Y | Y | U | N | Y | N/A |
| CR, Proietti et al. (2020) 24 | Y | Y | Y | Y | Y | Y | N/A | Y | - | - |
| CR, Rubin et al. (2020)15 | Y | Y | Y | Y | Y | Y | N/A | Y | - | - |
| CR, Santonja et al. (2020)13 | Y | Y | Y | Y | Y | Y | N/A | Y | - | - |
| CR, Shah et al. (2021)20 | Y | Y | Y | Y | Y | Y | N/A | Y | - | - |
| CR, Wee and Tey (2020)19 | Y | Y | Y | Y | Y | Y | N/A | Y | - | - |
Results of Joanna Briggs Institute Critical Appraisal Checklists for Cross-Sectional and Cohort studies.
| Study type, Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional, Brancaccio et al. (2021) 22 | Y | Y | Y | Y | N | N | Y | N/A | - | - | - |
| Cross-sectional, Mendez-Maestro et al. (2020)18 | Y | Y | Y | Y | N | N | Y | Y | - | - | - |
| Retrospective cohort, Recalcati et al. (2021)16 | Y | Y | Y | N | N | N | Y | N/A | N/A | N/A | Y |
| Prospective cohort, Rekhtman et al. (2021)17 | Y | Y | Y | N | N | N | Y | Y | Y | N/A | Y |
The included studies yielded information on 29 patients. Sex and specific ages were evaluable for eleven of the thirteen studies (19 cases). There were 8 males and 11 females. Ages ranged from 19–82 years. The remaining studies provided age ranges for their entire cohorts and minimum (55) and maximum (77) ages could be deduced. Race was generally unreported. Regarding geographic distribution, four studies included nine patients exclusively from United States of America,15, 17, 20, 25 while six studies (13 patients) were reported from continental Europe (Spain, Germany and Italy).13, 16, 18, 22–24 Four patients were collaboratively reported between the United States of America and Brazil,14 one study detailing 2 patients from Qatar17 and a single patient was reported from Southeast Asia (Singapore).19
Regarding clinical presentation, twelve studies reported hospitalization status;13–24 15 outpatient and 16 inpatient cases were reported (unreported in one study of three patients).25 Details regarding temporal relationship of the eruption to the overall course of disease was available for 9 cases with pseudo-chilblains occurring on day 1 (n=3), day 3 (n=1), day 13 (n=1), 2 weeks (n=2), 3 weeks n=1) and 6 weeks (n=1) after onset of other COVID-19 related symptoms.13, 14, 16, 20, 22–24 Exposure to cold/damp was excluded in four studies, (10/29 cases) and unreported in the remainder.15, 16, 18, 20 Anatomical locations included toes/feet, hand/fingers, ears, arms and legs. 28/29 patients had involvement of hands/feet/digits. There were two reports of ear involvement, one patient with an ear-only lesion.14, 24 Toes/feet were the most commonly reported single location. Chilblains-related symptomatology was reported in 21 patients (nine studies), with 7 experiencing symptoms (pain/pruritus/swelling) and 14 were asymptomatic. 14, 15, 18–24 Presence of extracutaneous symptoms of COVID-19 was evaluable for twelve studies. Although specific details were only provided for ten studies,13–16, 19–24 two studies were taken from inpatient cohorts of subjects admitted for COVID-19-related complications,17, 18 and so had extracutaneous features. One study did not comment on symptoms.25 Extracutaneous COVID-19 symptoms were experienced in 17 cases (including fever, headache, diarrhea, respiratory symptoms and sensory disturbances) and 9 cases lacked extracutaneous manifestations. Resolution timelines could be assessed in eight studies (13 cases). 13–16, 19, 20, 22, 24 Three cases resolved at < 7 days, 4 cases between 8–14 days, 2 cases between 15–21 days and 4 cases took >21 days (maximum of >50 days). Pseudo-chilblains management was detailed in eight studies with 2 patients receiving analgesics (non-steroidal anti-inflammatory drug and paracetamol), 1 receiving low molecular weight heparin and aspirin, 1 receiving heparin and methylprednisolone and 11 observed.13,16–20,22,23 Five studies (5 cases) highlighted the temporal relationship of pseudo-chilblains to COVID-19 testing; recognition of eruption triggered COVID-19 testing in 4 of these patients.13, 15, 19, 20,24
Biopsies were performed in five of 13 studies,13, 14, 16, 23, 25 although it was unclear whether all patients were sampled in two of these reports.14, 16 Two patterns were seen; 1) spongiotic/dyshidrotic dermatitis, necrotic keratinocytes and a superficial perivascular lymphocytic infiltrate and 2) a perivascular +/- periadnexal lymphocytic infiltrate. The latter pattern accounted for at least five cases.13, 16, 25 Immunohistochemistry/immunofluorescence was performed in three studies (5 cases) using antibodies against the COVID-19 spike protein (SARS-CoV/SARS-CoV-2 spike 1A9; GeneTex, Inc., Irvine, CA, USA and Sino Biological, 40?150-T62-COV) while ISH was concurrently performed in one paper (Advanced Cell Diagnostics anti-SARS-CoV-2 SP probe V-nCoV2019-S, performed on the Leica BOND-III platform, Wetzlar, Germany). 13, 23, 24 Although ISH was negative, IHC detected SARS-CoV-2 spike protein (granular staining pattern) localized to vascular endothelium in all five cases with concurrent eccrine gland positivity in 3 patients. Direct immunofluorescence performed in one patient revealed perivascular deposition of C3, C5b-9 and C1q. 13
While from an epidemiologic perspective, the rise in chilblain-like lesions during the onset of the COVID-19 pandemic points to an association with COVID-19, the lack of confirmatory testing is a significant limitation.2, 26–28 As in other viral eruptions (e.g., unilateral laterothoracic exanthem), numerous agents may produce similar findings and care must be taken in ascribing causality. Furthermore, the frequent lack histopathologic confirmation, variation in clinical appearance and microscopic features, and absence of clinical photographs for many reports raises the possibility that the designation pseudo-chilblains/''COVID-toes'' may represent a heterogenous group of conditions with similar anatomic distribution. This study aims to contribute to our evolving understanding of COVID-19-associated skin disease by specifically examining the features of pseudo-chilblains in adults from studies where patients were definitively infected. It should be noted a positive serologic test or RT-PCR for COVID-19 is not necessarily an indicator of active infection in otherwise asymptomatic patients, as both may remain positive for some time after infection. 29 Perhaps in some patients, pseudo-chilblains represent a delayed reaction to recent but inactive infection.30
Our analysis suggests that many reported cases of pseudo-chilblains do not detail laboratory confirmation of COVID-19 infection. In studies meeting our inclusion criteria, we found pseudo-chilblains in adults occurred in both sexes over a wide age range (2nd-9th decades). Most cases were reported from non-equatorial countries. The apparent geographic distribution and acral localization may implicate environmental factors as concomitant triggers.3
Pseudo-chilblains have been suggested as a marker for mild disease.4 While the number of cases evaluated in this study is too small to confirm or refute this, it is noteworthy that pseudo-chilblains occurred in both well outpatients and persons hospitalized with COVID-19 complications. 17, 18 While details of the onset of pseudo-chilblains relative to overall disease-course were not clear in most studies, where evaluable, pseudo-chilblains could occur from Day 1 of illness to six weeks from initial symptoms, suggesting its potential appearance in acute and more chronic phases of infection, or perhaps in patients with recent but inactive infection. Cold/damp exposure was excluded in 10/29 of the cases. Unfortunately, a history of previous conventional chilblains was generally unreported. Currently pathomechanistic similarities/differences of conventional and pseudo-chilblains are not known.
Pseudo-chilblains could be either asymptomatic or symptomatic. Extracutaneous symptoms were present in greater than two thirds of cases analyzed but no characteristic pattern could be elucidated with respiratory, sensory, gastrointestinal, headache and fever being represented. Resolution time was likewise heterogenous some patients resolving within a week and others longer up to 50 days. Therapeutic approach was not standard and included anti-inflammatory and analgesic agents, anticoagulants, and observation.
Regrettably, biopsies were not performed in the majority of cases examined nor in larger global registry reported cases.5 Reported histopathologic features include vacuolar change, spongiosis, necrotic keratinocytes, a superficial and deep perivascular and perieccrine lymphocytic/lymphohistiocytic infiltrate, lymphocytic vasculitis, subepidermal blister formation, papillary dermal edema, extravasation of erythrocytes, increased intradermal mucin and microthrombi.5,31 In our included cases, intraepidermal vesicular (dyshidrotic-like) dermatitis and a superficial and deep perivascular and perieccrine lymphocytic infiltrate were described. While further work outlining histopathologic changes is needed, a perivascular and periadnexal lymphocytic infiltrate similar to conventional chilblains appears to be common, though not universal.13, 25, 32 Interestingly, biopsies may aid in tissue-based confirmation of infection.25 In 4 out of 5 cases, COVID-19 spike protein was visualized via IHC/IF in vascular endothelium and in eccrine epithelium despite negative nasal PCR and/or serology. It is important to note that like nasal/nasopharyngeal RT-PCR and serology, spike protein identification may not equate to active infection. The spike protein is thought to be cleaved, entering endothelium/epithelium via the angiotensin converting enzyme type two receptor 25 but how long it remains within these cells is unclear.
Based on our analysis, features of classical chilblains and pseudo-chilblains in adults with confirmed COVID-19 infection were compared. Typical chilblains present with painful, acral, erythematous/livid lesions in young, predominantly female patients within the Northern Hemisphere after exposure to cold/damp conditions.33 Microscopic features include superficial and deep perivascular and perieccrine lymphocytic infiltrates, papillary dermal edema and extravasation of erythrocytes.34 Similarities include anatomical and perhaps geographic distribution, morphology and some histopathologic findings. Differences include the often asymptomatic nature, potential for chronicity, lack of exposure to cold/damp, variability in histopathologic findings and the occurrence over a broad age range in both sexes in COVID-19 related lesions compared with classical chilblains. Limitations to this study include the retrospective nature of systematic reviews, occasional methodologic gaps in some of the included studies and the exclusion of cases from large databases where confirmation of COVID-19 status was unavailable and where specific clinical data is often limited at best may have resulted in some true cases of COVID-19 related chilblains being unavailable for analysis.
Many patients reported as pseudo-chilblains of COVID-19 do not have confirmed infection. In adult patients with confirmed COVID-19, chilblain-like lesions have been reported primarily from North America and Europe, occur across the spectrum of age in males and females, favor acral surfaces, may be symptomatic or asymptomatic, lack relationship to cold/damp exposure, display variability in resolution time and association with extracutaneous COVID-19 manifestations, occurs in both well and ill patients and may serve as a trigger for COVID-19 testing. Histopathologic features resemble that of classical chilblains but less common patterns may occur. Further work is needed to clarify the relationship of acral eruptions and COVID-19. Infection confirmation, photographic documentation and histopathology are critical to establish homogeneity in reported pseudo-chilblains during this global pandemic.
Many organs can be affected by infection with COVID-19. The skin is no different. One of the earliest skin signs of COVID-19 infection was labeled “COVID-toes”, where patients get red-to-purple spots/rashes, primarily on their toes or fingers. In the dermatology world, the preferred name for ‘'COVID-toes'' is ‘pseudo-chilblains' referencing the similarity in appearance of the rash to a condition called chilblains affecting fingers and toes of people who have been exposed to cold and wet conditions for a relatively prolonged time. While little doubt exists that this peculiar rash may be a manifestation of infection with COVID-19, we were struck by the fact that many of the reported cases did not have confirmed infection. In the future, as we look back at the science and data generated during this period, the lack of laboratory confirmation of infection may render some of the conclusions drawn invalid, or at least uncertain. We wished to examine the clinical and laboratory characteristics of adult patients with COVID-toes (pseudo-chilblains) with confirmed infection.
To do this, we performed a systematic review of the published literature on the PubMed/Medline database following the standard guidelines for this type of research (Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PRISMA). We used studies reporting adults (>18 years) with confirmed COVID-19. We recorded the type of study performed, which country the patients came from, age, sex and race of the patients reported, how close the onset of COVID-toes was to the diagnosis of COVID-19 infection, the type of testing used to confirm infection, whether the patient was kept in hospital or not, where on the body the rash occurred, whether the patient had a history of being exposed to cold or wet conditions, whether the rash had any symptoms, whether the patients had any non-skin manifestations of COVID-19 infection, how long the rash took to go away and what treatment if any was prescribed to patients with COVID-toes. We also documented if small pieces of skin were taken (biopsies) to describe what the rash looks like microscopically.
Our search identified only 13 studies giving us details on 29 patients. In COVID-19-infected adults, ‘'COVID toes'' were most commonly reported from North America and Europe, occurred in both males and females over a wide age-range. Both well people and ill patients who were admitted to hospital could be affected. The hands and feet were most commonly affected but lesions on the ear could also be seen. ‘'COVID-toes'' could be symptomatic or not. Many patients had evidence of COVID-19 infection besides rash (e.g. cough or diarrhea). ‘'COVID-toes'' could take <1 week or up to greater than 50 days to resolve. No standard treatment for the rash was found. Biopsies are infrequently performed but when done, findings similar to classical chilblains are described.
In summary, many patients reported as pseudo-chilblains of COVID-19 do not have confirmed infection. Infection confirmation, photographs and biopsies are recommended if we are to be sure that every person reported as “COVID-toes” has the same rash. Further work clarifying the relationship of rashes on the hands and feet with COVID-19 infection is necessary.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: MGW, JDH. Methodology: MGW JDH. Formal Analysis: MGW JDH. Data Curation: SH, KW, NT, JM, MGW, JDH. Resources: MGW, JDH. Writing – Original Draft: SH, JDH. Writing – Review & Editing: SH, KW, NT, JB, MGW, DB, JDH. Visualization: MGW, DB, JDH. Supervision: MGW, JDH. Project Administration: MGW, JDH.
1. Gupta S, Gupta N, Gupta N. Classification and pathophysiology of cutaneus manifestations of COVID-19. Int J Res Dermatol. 2020;6(4):1–5.
2. Galván Casas C, Catala A, Carretero Hernández G, RodríguezíJiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–7.
3. AlMahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10(2):128–35.
4. Ghazal S, Litvinov IV, Aljahani N, Jfri A, Netchiporouk E. Cutaneous manifestations of coronavirus disease 2019 (COVID-19) infection-what do we know so far. J Cutan Med Surg. 2020;24(4):416–7.
5. Freeman EE, McMahon DE, Lipoff JB, Rosenbach M, Kovarik C, Takeshita J, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486–92.
6. Frumholtz L, Bouaziz JD, Battistella M, et al. Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185(6):1176–85.
7. Gómez-Fernández C, López-Sundh AE, González-Vela C, et al. High prevalence of cryofibrinogenemia in patients with chilblains during the COVID-19 outbreak. Int J Dermatol. 2020;59(12):1475–84.
8. Gao JC, Huang A, Desai A, Safai B, Marmon S. “COVID toes”: A true viral phenomenon or a diagnosis without a leg to stand on?. JAAD Int. 2022;9:1–6.
9. Le Cleach L, Dousset L, Assier H, Fourati S, Barbarot S, Boulard C, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183(5):866–74.
10. Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., … & Stewart, L. A. (2015), Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
11. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute. 2017. Available from https://reviewersmanual.joannabriggs.org/
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
13. Santonja C, Heras F, Núñez L, Requena L. COVID-19 chilblain-like lesion: immunohistochemical demonstration of SARS-CoV-2 spike protein in blood vessel endothelium and sweat gland epithelium in a PCR-negative patient. Br J Dermatol. 2020;183(4):778–80.
14. Almeida G, Arruda S, Marques E, Michalany N, Sadick N. Presentation and Management of Cutaneous Manifestations of COVID-19. J Drugs Dermatol. 2021:76–83.
15. Rubin A, Alamgir M, Rubin J, Rao BK. Chilblain-like lesions with prominent bullae in a patient with COVID-19. BMJ Case Reports CP. 2020;13(11):e237917.
16. Recalcati S, Gianotti R, Fantini F. COVID-19: The experience from Italy. Clin Dermatol. 2021;39(1):12–22.
17. Rekhtman S, Tannenbaum R, Strunk A, Birabaharan M, Wright S, Grbic N, et al. Eruptions and related clinical course among 296 hospitalized adults with confirmed COVID-19. J Am Acad Dermatol. 2021;84(4):946–52.
18. Mendez Maestro I, Pena Merino L, Udondo Gonzalez del Tanago B, Aramburu González A, Orbea Sopeña A, Sanchez De Vicente J, et al. Skin manifestations in patients hospitalized with confirmed COVID-19 disease: a cross-sectional study in a tertiary hospital. Int J Dermatol. 2020;59(11):1353–7.
19. Wee C, Tey HL. Chilblain-like eruption in COVID-19 disease: possible pathogenetic role of temperature. Eur J Dermatol. 2020;1(1).
20. Shah I, Stacey SK, Ganne N, Merfeld J. Perniosis in the COVID-19 era. Dermatol Online J. 2021;27(5).
21. Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45(6):746–8.
22. Brancaccio G, Gussetti N, Sasset L, Alaibac M, Tarantello M, Salmaso R, et al. Cutaneous manifestations in a series of 417 patients with SARS-CoV-2 infection: epidemiological and clinical correlates of chilblain like lesions. Pathog Glob Health. 2021;115(7–8):483–6.
23. Gambichler T, Reuther J, Stücker M, Stranzenbach R, Torres-Reyes C, Schlottmann R, et al. SARS-CoV-2 spike protein is present in both endothelial and eccrine cells of a chilblain-like skin lesion. J Eur Acad Dermatol Venereol. 2021;35(3):e187–9.
24. Proietti I, Tolino E, Bernardini N, Mambrin A, Balduzzi V, Marchesiello A, et al. Auricle perniosis as a manifestation of Covid-19 infection. Dermatol Ther. 2020;33(6):e14089.
25. Ko CJ, Harigopal M, Gehlhausen JR, Bosenberg M, McNiff JM, Damsky W. Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2021;48(1):47–52.
26. Magro C, Mulvey J, Laurence J, Sanders S, Crowson A, Grossman M, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184(1):141–50.
27. Gómez-Fernández C, López-Sundh AE, González-Vela C, Ocejo-Vinyals JG, Mayor-Ibarguren A, Salas-Venero CA, et al. High prevalence of cryofibrinogenemia in patients with chilblains during the COVID-19 outbreak. Int J Dermatol. 2020;59(12):1475–84.
28. McCleskey PE, Zimmerman B, Lieberman A, Liu L, Chen C, Gorouhi F, et al. Epidemiologic analysis of chilblains cohorts before and during the COVID-19 pandemic. JAMA Dermatol. 2021;157(8):947–53.
29. Zhang L, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci U S A. 2021;118(21).
30. Gisondi P, PIaserico S, Bordin C, Alaibac M, Girolomoni G, Naldi L. Cutaneous manifestations of SARS-CoV-2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020;34(11):2499–504.
31. Lee DS, Mirmirani P, McCleskey PE, Mehrpouya M, Gorouhi F. Cutaneous manifestations of COVID-19: a systematic review and analysis of individual patient-level data. Dermatol Online J. 2020;26(12).
32. Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): Histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83(3):870–5.
33. Goette DK. Chilblains (perniosis). J Am Acad Dermatol. 1990;23(2):257–62.
34. Ko CJ, Harigopal M, Damsky W, Gehlhausen JR, Bosenberg M, Patrignelli R, et al. Perniosis during the COVID-19 pandemic: Negative anti-SARS-CoV-2 immunohistochemistry in six patients and comparison to perniosis before the emergence of SARS-CoV-2. J Cutan Pathol. 2020;47(11):997–1002.
Seon Hayles, 1 BSc. Fifth-year Medical Student. The University of the West Indies, Mona Campus, Jamaica W.I.
Kelsey Williams, 1 BSc. Fifth-year Medical Student. The University of the West Indies, Mona Campus, Jamaica W.I.
Nidhi Thomas, 1 BSc. Fifth-year Medical Student. The University of the West Indies, Mona Campus, Jamaica W.I.
Jabari Morgan, 1 BSc. Fifth-year Medical Student. The University of the West Indies, Mona Campus, Jamaica W.I.
Donna Braham, 2 MBBS, DM. Section of Dermatology, The University of the West Indies, Mona Campus, Jamaica W.I.
Maxine Gossell-Williams, 3 PhD. Section of Pharmacology and Pharmacy, Department of Basic Medical Sciences, The University of the West Indies, Mona Campus, Jamaica W.I.
Jonathan D. Ho, 4 MBBS, D.Sc. Section of Dermatology and Department of Pathology, The University of the West Indies, Mona Campus, Jamaica W.I.
About the Author: Seon Hayles is a fifth-year medical student at The University of the West Indies, Mona Campus, Kingston Jamaica.
Correspondence: Jonathan D. Ho. Address: University of the West Indies, Mona Campus, Kingston, Jamaica. Email: jdho@bu.edu
Editor: Francisco J. Bonilla-Escobar; Student Editors: Bahadar Srichawla & Nikoleta Tellios; Copyeditor: Michael V. Tavolieri; Proofreader:Amy Phelan; Layout Editor: Ana Maria Morales; Process: Peer-reviewed
Cite as Hayles S, Williams K, Thomas N, Morgan J, Braham D, Williams MG, et al. Pseudo-Chilblains in Adult Patients with Confirmed COVID-19: A Systematic Review. Int J Med Stud. 2023 Jul-Sep;11(3):220-8.
Copyright © 2023 Seon Hayles, Kelsey Williams, Nidhi Thomas, Jabari Morgan, Donna Braham, Maxine Gossell-Williams, Jonathan D. Ho
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 11, NUMBER 3, August 2023