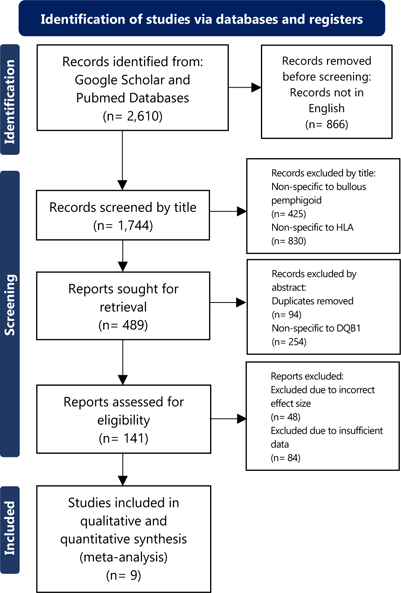
PRISMA Flow Diagram for HLA-DQB1*0301 in Bullous Pemphigoid
Dylan Thibaut1, Ryan Witcher1, Breana Barnes1, Kersten T. Schroeder2
doi: http://dx.doi.org/10.5195/ijms.2023.1594
Volume 11, Number 3: 199-205
Received 30 06 2022; Rev-request 15 08 2022; Rev-request 01 03 2023; Rev-recd 16 10 2022; Rev-recd 11 05 2023; Accepted 13 09 2023
ABSTRACT
Background:The linkage of HLA-DQB1*0301 to autoimmune disorders is becoming more common in literature. Despite bullous pemphigoid (BP) and pemphigus vulgaris (PV) both having similar symptoms, such as blistering skin conditions, research has shown different relationships with HLAs.
Methods:In this systematic review, HLA-DQB1*0301 and the odds of developing BP and PV were explored. Google Scholar and Pubmed were consulted, and articles were included if living subjects were used, odds ratio was available or could be ascertained from the study, and if it was not a meta-analysis of other researcher's works. MetaXL software was used to generate data for analysis and a forest plot was generated for each. Nine studies conducted between 1996 and 2021 met study selection criteria for the BP HLA-DQB1*0301 meta-analysis (1,340 patients and 6,673 controls) and five studies (247 patients and 2,435 controls) for PV.
Results:HLA-DQB1*0301 increased the odds of developing BP (OR= 1.64, 95% CI [1.44, 1.87], I2= 0%) yet decreased odds of PV (OR= 0.60, 95% CI [0.40, 0.89], I2= 34%).
Conclusion:Results suggest HLA-DQB1*0301 may serve opposite roles in BP and PV despite similarity in symptoms, finding higher odds for developing BP versus lower odds for developing PV. Understanding this HLA's function in each requires further exploration. Limitations of the analysis included minor asymmetry in the PV Doi plot, suggesting publication bias. No funding was used; study protocol was not registered.
Keywords: HLA-DQB1 antigen; Pemphigus; Pemphigoid; Bullous; DQB1*0301; Bullous pemphigoid; Bullous pemphigus; Dermatology; Skin; Vesicles; Skin layer; Autoimmunity; Inflammation; Inflammatory syndrome; Pemphigoids; Skin disease; Vesiculobullous Skin Diseases; Autoimmune Diseases; Immune System Diseases (Source: MeSH-NLM).
Autoimmune blistering diseases are conditions in which autoantibodies form against the dermal or subdermal layers of skin, causing damage to the skin. The most common of these conditions include bullous pemphigoid (BP) and pemphigus vulgaris (PV), with each having a different target by which autoantibodies attack despite having a similar immunologic mechanism. Both conditions occur through a T2 hypersensitivity reaction.1 This reaction occurs through a series of steps: human antigens are taken up by major histocompatibility complexes (MHCs) and are incorrectly seen as foreign, antibodies are formed against normal human substances, the antibodies attach to the human antigens they recognize, and the autoantibodies attached to human antigen cause immune cells to attack the human tissue. This process revolves around human leukocyte antigens (HLA), which are variable genes encoding MHCs. With a vast variance in HLAs across populations and with humans inheriting multiple from each parent, immunity across different individuals differs greatly.
In BP, a T2 hypersensitivity reaction occurs where IgG anti-hemidesmosome antibodies are directed towards the hemidesmosomal proteins BPAg1 and BpAg2.2 Hemidesmosomes connect cells to the basement membrane below. The result of autoantibody attack of hemidesmosomes in BP is the formation of large and rigid subepidermal blisters which rarely rupture that primarily affect palms, soles, groin, and axillae.3 BP is the most common of the blistering skin conditions, with a peak incidence of >60 years of age. PV instead uses IgG anti-desmosomal antibodies which target desmoglein 3 and desmoglein 1.4 Desmosomes are responsible for connecting cells in the epithelium to one another, unlike hemidesmosomes, which connect the cells to the basement membrane below. The result of the autoantibody attack in PV is the formation of smaller and fragile intraepidermal blisters that frequently rupture and crust.3 These blisters primarily affect intertriginous areas, with a unique perioral involvement.
Understanding the specific HLAs that lead to this autoimmune process is essential to understanding BP and PV development, progression, and treatment. Presently, the combined prevalence of HLA research on BP and PV has been centered on HLA-DQB1 allele variances. HLA-DQB1 has been associated with several pathologies including susceptibility to Type 1 Diabetes Mellitus (T1DM), as well as superimposed BP when T1DM patients are treated with DPP4i.5–6 In association with other alleles in linkage disequilibrium, it has shown to be related to rheumatoid arthritis as well as multiple sclerosis.7 Research specific to HLA alleles has shown that each allele, even if similar, can have a different effect on autoimmunity development and bodily response.
HLA-DQB1*0301 is one specific allele with implications to care, as it is connected to prognosis, treatment response, and even symptom profile in dermatologic conditions.8–10 This is apparent with BP and PV, which are also seemingly connected to the allele.10–11 With this allele being mentioned in both skin conditions as showing opposing effects of protecting versus predisposing patients to PV and BP, a meta-analysis is necessary. Through a better understanding of the effects of this specific allele, the connection between it and these conditions can further be understood and potential medical applications for immunologic methods can be deduced. This systematic review hopes to find the connection between HLA-DQB1*0301 alleles and the odds of BP and PV to better highlight the alleles and their role in disease pathophysiology.
Systemic reviews were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.12 Eligibility criteria for this study included a requirement that all subjects to be living and all studies to be in the English language. All included studies were required to be case-control studies. Each included study required control samples and case samples to be from the same population. Case samples were required to either be diagnosed patients with BP or PV to be compared. Exclusions for this study include the use of subjects that are classified as deceased, animal, or cell subjects or samples. No meta-analyses were included in this study as part of the analysis. The databases used for this meta-analysis were Google Scholar and PubMed. The search for each meta-analysis used the following exact terms: “HLA-DQB1*0301”, “HLA-DQB1*0301 bullous pemphigoid”, “HLA-DQB1*0301 pemphigus vulgaris.” The protocol was agreed upon prior to beginning the analysis consistent with the methods described here.
The search for articles began May 2021. The selection process was conducted through two researchers independently searching for and finding studies with the oversight of a PhD principal investigator The search strategy was designed by the principal investigator in which two researchers collected data and sifted through search results. First, the search terms were used on the databases, followed by removal of all studies using a language other than English through search tools that are part of the databases. This was followed by compiling search results on a shared document based on the title. After this, results were filtered out if they did not meet inclusion criteria. No specific filter software was used. The variables gathered for assessment were the odds ratio, lower and upper range for confidence intervals (CI), number of patients, control group, as well as the demographic groups within the publication. If an odds ratio was not provided, but sufficient data was present, this data was used to calculate the odds ratio. All data was compiled on a shared document where either another researcher would agree to including a study or disagree with its inclusion. If disagreement occurred, the final decision was made by the principal investigator. Database search results and study evaluation for inclusion criteria are indicated in Figure 1 and Figure 2.
PRISMA Flow Diagram for HLA-DQB1*0301 in Bullous Pemphigoid

PRISMA Flow Diagram for HLA-DQB1*0301 in Pemphigus Vulgaris.

Luis Furuya-Kanamori index (LFK index) was determined and a Doi plot was created for each of the analysis as a means of assessing publication bias.13 Doi plots combined with LFK index allows for a quantitative and visual representation of bias. The Doi plot represents included studies in a graph form while LFK index measures the asymmetry of the created plot. Ideally, plots should show no asymmetry or minor asymmetry (a value between -1 to +1), with larger values indicating inconsistency across included studies. The inclusion of Doi plot and LFK index provides an additional layer to determine consistency of findings across studies.
Additionally, the NIH Quality Assessment Tool was used to evaluate individual studies included in the analysis; if a particular study was found to have a majority of the questions on the bias assessment as “no”, it was specifically removed for inclusion and mentioned.14 The effect size was measured using the odds ratio, no other effect sizes were included in the meta-analysis. When determining the eligibility of each study used in the meta-analysis, studies that provided enough data to calculate the effect size were included and used to calculate the odds ratio. Data collected from studies that failed to provide sufficient data to calculate effect size or those who did not meet inclusion criteria were not included in the study. Data was collected by researchers and compared; this data was then combined into a table to visually display the results of individual studies. In order to synthesize results, MetaXL software was used to generate a forest plot and conduct the meta-analysis.15 This analysis used the IVhet model. Heterogeneity was calculated using Cochran's Q and an I2 value. A result with a P value <0.05 or an I2 value greater than or equal to 25% were significant for heterogenicity. Sensitivity testing was performed using pooled odds ratios and subgroup analysis.16 To report bias assessment, literature heterogeneity was noted in the results. The odds ratio obtained from the forest plot analysis as well as its 95% confidence interval were used to evaluate the outcome in this study. All research was conducted under appropriate ethical guidelines for research set by the institution the research was performed at.
Nine studies conducted between 1996 and 2021 met study selection criteria for the BP HLA-DQB1*0301 meta-analysis.7, 17–24 One study seemingly met all criteria for the BP analysis, though due to its usage of deceased patients as a control group, was excluded due to selection criteria not allowing animals, deceased patients, or microorganisms as a studied sample for comparison.25 A combined total of 1,340 patients with BP and 6,673 controls were included from the nine studies. Several different demographic groups were examined in these studies including German, Caucasian, Han Chinese, Japanese, Iranian, and Northern Chinese. For the PV analysis, five studies conducted between 1999 and 2021 met selection criteria.26–30 Despite meeting most inclusion criteria, one study was removed from the PV analysis due to its usage of animals, deceased patients, or microorganisms as a sample for comparison.31 Due to exclusion criteria prohibiting past meta-analyses to be incorporated into this study's meta-analysis, an additional study was also removed.32 A total of 247 PV patients and 2,435 controls were used in this five-study analysis. Demographic groups included in these studies consisted of Vietnamese, Serbian, Slovak, Venezuelan, and Italian groups.
Odds ratio, 95% confidence interval, size of patient sample, size of the control sample, and the group being tested were recorded for all studies used for the meta-analyses. Recorded details collected from the studies of both analyses are summarized in Table 1. To test for publication bias, a Doi plot was generated for each analysis. The BP analysis showed no asymmetry with an LFK index of 0.83. Minor asymmetry was found in the PV analysis, with an LFK index of -1.43.
Characteristics of Studies of HLA-DQB1*0301 in Bullous Pemphigoid and Pemphigus Vulgaris.
| Author | OR | CI lower | CI upper | Patient | Control | Group |
|---|---|---|---|---|---|---|
| Bullous Pemphigoid | ||||||
| Schwarm et al., 2021 | 1.84 | 1.01 | 3.35 | 446 | 433 | German |
| Lindgren et al., 2019 | 2.76 | 1.42 | 5.35 | 23 | 2991 | Caucasian |
| Fang et al., 2018 | 1.69 | 1.19 | 2.39 | 105 | 420 | Han Chinese |
| Sun et al., 2018 | 1.58 | 1.33 | 1.88 | 575 | 976 | Han Chinese |
| Ujiie et al., 2018 | 1.60 | 0.90 | 2.90 | 72 | 873 | Japanese |
| Esmaili et al., 2013 | 1.82 | 1.13 | 2.92 | 50 | 180 | Iranian |
| Gao et al., 2002 | 1.29 | 0.50 | 3.31 | 25 | 57 | Chinese |
| Okazaki et al., 2000 | 1.48 | 0.57 | 3.85 | 23 | 525 | Japanese |
| Delgado et al., 1996 | 1.38 | 0.70 | 2.70 | 21 | 218 | Caucasian |
| Pemphigus Vulgaris | ||||||
| Vuong et al., 2021 | 1.05 | 0.49 | 2.11 | 22 | 101 | Vietnamese |
| Zivanovic et al., 2016 | 0.42 | 0.21 | 0.85 | 72 | 1992 | Serbian |
| Párnická et al., 2013 | 0.65 | 0.34 | 1.25 | 43 | 113 | Slovakian |
| Sáenz-Cantele et al., 2007 | 0.25 | 0.09 | 0.73 | 49 | 101 | Venezuelan |
| Lombardi et al., 1999 | 0.66 | 0.36 | 1.22 | 61 | 128 | Italian |
To calculate a combined odds ratio for the various studies, an inverse variance heterogeneity (IVhet) model was used via MetaXL software to generate corresponding forest plots (Figure 3).15 Odds of BP was higher given a person had HLA-DQB1*0301 (OR= 1.64, 95% CI [1.44, 1.87], I2= 0%) while odds of PV was lower given the person had HLA-DQB1*0301 (OR= 0.60, 95% CI [0.40, 0.89], I2= 34%). This finding suggests that HLA-DQB1*0301 has opposite effects in each condition, increasing odds of BP while reducing odds of PV. Note that the heterogeneity of the PV analysis is above 25% and should be interpreted with a level of caution that some studies may have influenced the results (I2= 34%).
Forest Plot for Bullous Pemphigoid (Top) and Pemphigus Vulgaris (Bottom).

Sensitivity testing was performed via the exclusion of each study individually and sequentially. For the BP analysis, two studies contributed most to the pooled OR.18, 19 No substantial heterogeneity result was found on analysis, and I2=0.0 for all studies analyzed for it. Regarding the PV analysis, two studies were found to most effect pooled OR.26, 28 Heterogeneity testing in this case showed two values to note: Párnická et al.'s I2= 50.11 and Lombardi et al.'s I2= 49.65.27, 29 Though no Q value was considered significant for these two studies, minor asymmetry found in the Doi plot was a result.
Despite the relative similarity of BP and PV, the findings of this meta-analysis support that HLA-DQB1*0301 has opposing effects in each condition. While it was found to increase odds of BP in those with HLA-DQB1*0301 (OR= 1.64, 95% CI [1.44, 1.87]), there are decreased odds of PV in those with the HLA (OR= 0.60, 95% CI [0.40, 0.89]). With this finding of opposing results in these conditions, a question as to why there are differing effects despite similarity in symptoms must be asked.
With HLA-DQB1*0301 being more common in BP according to this study's findings, there is a need to understand what to expect this association to mean clinically. Regarding autoimmune consequence parallels in other autoimmune conditions, the DQB1*0301 allele was found to be associated with more severe outcomes in patients with multiple sclerosis, type 1 diabetes mellitus and celiac disease.33–35 Regarding DQB1*0301 association with skin disorders, it was found to be correlated with the development of cutaneous melanoma, erythema multiforme, as well as ocular cicatricial pemphigoid.36–38 Membranous pemphigoid also shows this increase as well.39 It has been postulated that the HLA class II antigen presentation seen in higher amounts within keratinocytes may stimulate a T-cell inflammatory response, contributing to the increased susceptibility of skin disorders with the HLA-DQB1*0301 allele.38
PV did not follow this deleterious effect, instead showing an opposite, seemingly protective role. Unfortunately, the heterogeneity of I2= 34% calls into question whether this finding is a result of not including a sufficient number of studies. A more global inclusion of different samples may help understand this connection more completely. Other epithelial tissues outside of blistering skin conditions have shown a potential effect, such as in gastric cancer.40 Autoimmune disorders that are not skin related, such as autoimmune hepatitis, similarly show HLA-DQB1*0301 as protective.41
Among multiple autoimmune diseases, there is a diverse set of HLA antigens with an association yet to be discovered. Clinically in the future, HLA antigen sequencing could perhaps be a standard of care in diagnosing new patients. This data may lead to the ability to increase the pathological predictive ability of medical geneticists in the future.
The meta-analysis conducted to evaluate the impact of HLA-DQB*10301 on the prevalence of BP and PV considered various variables and limitations of the pooled studies. However, it is important to acknowledge certain strengths and limitations of this analysis. While the meta-analysis involved a comprehensive approach by including multiple studies, no single methodology encompassed a representative global population. Instead, individual studies primarily focused on specific ethnicities, leading to variations in the significance of the results. The study utilized strict inclusion and exclusion criteria, with pre-defined methods of data utilization and data storage which would help avoid post-hoc analysis bias.
However, the analysis was primarily conducted by two medical student researchers with PhD oversight, and thus the study lacked a dedicated statistician. Regarding the quality of the genes analyzed, the majority of the studies concentrated solely on the effect of a single haplotype on BP and PV prevalence, with limited exploration of the effects of linkage disequilibrium or the combined impact of HLA allele frequencies on disease prevalence. Consequently, these factors impose limitations on the extent to which the current analysis can accurately predict the global impact of HLA-DQB1*0301. Furthermore, the reliance on only two broad databases restricts the generalizability and applicability of the research findings.
While heterogeneity was 0% for the BP comparison, it was a factor influencing the PV analysis (I2= 34%), making the result of the PV analysis more challenging to interpret. Additionally, while bias assessment found the included studies to overall be well designed, there was minor asymmetry in LFK index findings for the PV analysis. Both the heterogeneity findings and this minor asymmetry suggest that some of the included studies in the PV analysis may not have been as high quality. This may have influenced the findings.
Autoimmune blistering diseases, such as BP and PV, involve the formation of autoantibodies against the skin, leading to damage. These conditions are characterized by a T2 hypersensitivity reaction, with each condition targeting different proteins despite a similar immunologic mechanism. The human leukocyte antigen (HLA) genes, particularly HLA-DQB1*0301 allele, have been associated with various pathologies. Understanding the role of HLA alleles is crucial for understanding the development and treatment of BP and PV. This analysis included nine studies for BP and five studies for PV which collectively showed that HLA-DQB1*0301 allele increased the odds of BP and reduced the odds of PV. These findings suggest that HLA-DQB1*0301 has opposing effects in these two conditions. However, caution should be exercised due to some heterogeneity in the PV analysis. Further research is needed to explore the specific mechanisms underlying these associations and their implications for the diagnosis and treatment of BP and PV.
Title: HLA-DQB1*0301 in Bullous Pemphigoid and Pemphigus Vulgaris: A Meta-Analysis
Main problem to solve: Human Leukocyte Antigen (HLA) is something which takes up parts of the environment around or inside of cells. These parts, antigens, are then shown to immune cells by the HLA. Some specific types of these HLA have been linked to autoimmune problems. Bullous pemphigoid (BP) and pemphigus vulgaris (PV) are two examples of autoimmune blistering skin problems. These conditions both cause similar symptoms. Despite being similar, research shows that different HLAs are linked to each. Some HLAs can make it more likely to get one of the blistering skin problems and some HLAs can make it less likely to get one of the blistering skin problems. One specific HLA, HLA-DQB1*0301, has increased odds of certain autoimmune skin problems and has decreased odds of other autoimmune skin problems.
Aim of study: In this study, the odds of bullous pemphigoid and pemphigus vulgaris depending on whether a patient has HLA-DQB1*0301 is found through a meta-analysis. This is done so that the relationship between these autoimmune conditions and HLA-DQB1*0301 can be found.
Methodology: In this systematic review, HLA-DQB1*0301 and the odds of developing bullous pemphigoid and pemphigus vulgaris were explored. Google Scholar and Pubmed were used for searching. Articles were used if they had living subjects only, odds ratio was in the study or could be found from the study, and if the study was not a meta-analysis. MetaXL software was used to make a forest plot for bullous pemphigoid and for pemphigus vulgaris. Nine studies that were done between 1996 and 2021 had the required data for the bullous pemphigoid HLA-DQB1*0301 meta-analysis (1,340 patients and 6,673 controls) and five studies (247 patients and 2,435 controls) had the required data for pemphigus vulgaris meta-analysis.
Results: HLA-DQB1*0301 increased the odds of developing bullous pemphigoid (OR= 1.64, 95% CI [1.44, 1.87], I2= 0%). HLA-DQB1*0301 decreased the odds of pemphigus vulgaris (OR= 0.60, 95% CI [0.40, 0.89], I2= 34%).
Conclusion: Results suggest that HLA-DQB1*0301 has opposite effects in bullous pemphigoid and pemphigoid. There are increased odds for getting bullous pemphigoid and lower odds for getting pemphigus vulgaris if a patient has HLA-DQB1*0301.
We would like to thank Lake Erie College of Osteopathic Medicine, the University of Central Florida, and the 2021 Student-Run LECOM Student Research Association (SRA) Summer Internship Program for their support of this research.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization, Methodology, Software, Formal Analysis: DT. Investigation: DT, BB. Writing - Original Draft: DT, RW, BB. Writing - Review & Editing: DT, RW, KTS. Visualization: DT, RW. Project Administration: DT, KTS. Funding Acquisition: KTS.
1. InStatPearls. Type II Hypersensitivity Reaction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563264/. Last Update: July 4, 2023.
2. Moro F, Fania L, Sinagra JL, Salemme A, Di Zenzo G. Bullous pemphigoid: trigger and predisposing factors. Biomolecules. 2020;10(10):1432.
3. Di Lernia V, Casanova DM, Goldust M, Ricci C. Pemphigus vulgaris and bullous pemphigoid: update on diagnosis and treatment. Dermatol Pract Concept. 2020;10(3):e2020050.
4. Kershenovich R, Hodak E, Mimouni D. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev. 2014;13(4–5):477–81.
5. Rabbani A, Abbasi F, Taghvaei M, Rabbani B, Moradi B, Shakiba Y, Rezaei N, Amirzargar A. HLA-DRB,-DQA, and DQB alleles and haplotypes in Iranian patients with diabetes mellitus type I. Pediatr Diabetes. 2013;14(5):366–71.
6. Chouchane K, Di Zenzo G, Pitocco D, Calabrese L, De Simone C. Bullous pemphigoid in diabetic patients treated by gliptins: the other side of the coin. J Transl Med. 2021;19:1–0.
7. Fang H, Shen S, Zheng X, Dang E, Zhang J, Shao S, Qiao P, Li Q, Wang H, Li C, Sun L. Association of HLA class I and class II alleles with bullous pemphigoid in Chinese Hans. J Dermatol Sci. 2018;89(3):258–62.
8. Bateman AC, Turner SJ, Theaker JM, Howell WM. HLA-DQB1* 0303 and* 0301 alleles influence susceptibility to and prognosis in cutaneous malignant melanoma in the British Caucasian population. Tissue Antigens. 1998;52(1):67–73.
9. Gheorghe LM, Rugina S, Dumitru IM, Franciuc I, Martinescu A, Nastase V, Petcu LC, Constantinescu I. Association of HLA-DQB1 alleles with interferon/ribavirin therapy outcomes in a Romanian patient group infected with hepatitis C virus genotype 1b. J Cont Clin Pract. 2016;2(2):50–9.
10. Alpsoy E, Akman-Karakas A, Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Archives of dermatological research. 2015;307:291–8.
11. Broussard KC, Leung TG, Moradi A, Thorne JE, Fine JD. Autoimmune bullous diseases with skin and eye involvement: Cicatricial pemphigoid, pemphigus vulgaris, and pemphigus paraneoplastica. Clin Dermatol. 2016;34(2):205–13.
12. Moher D, Altman DG, Tetzlaff J. PRISMA Preferred Reporting items for systematic reviews and Meta-Analyses. Guidelines for Reporting Health Research: A User's Manual. 1996;1999:250.
13. Furuya-Kanamori L, Barendregt JJ, Doi SA. A new improved graphical and quantitative method for detecting bias in meta-analysis. JBI Evid. 2018;16(4):195–203.
14. National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Accessed: June 6, 2021. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed: May 4, 2021.
15. Barendregt JJ, Doi SA. MetaXL user guide, EpiGear International Pty Ltd, Sunrise Beach, Queensland, Australia; 2016.
16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
17. Schwarm C, Gola D, Holtsche MM, Dieterich A, Bhandari A, Freitag M, Nürnberg P, Toliat M, Lieb W, Wittig M, Franke A. Identification of two novel bullous pemphigoid-associated alleles, HLA-DQA1* 05: 05 and-DRB1* 07: 01, in Germans. Orphanet J Rare Dis. 2021;16(1):1–5.
18. Lindgren O, Varpuluoma O, Tuusa J, Ilonen J, Huilaja L, Kokkonen N, Tasanen K. Gliptin-associated Bullous Pemphigoid and the Expression of Dipeptidyl Peptidase-4/CD26 in Bullous Pemphigoid. Acta Derm Venereol. 2019;99(6):602–9.
19. Sun Y, Liu H, Wang Z, Wang C, Mi Z, Sun L, Bao F, Yu G, Zhou G, Zhang F. The HLA-DQB1* 03: 01 is associated with bullous pemphigoid in the Han Chinese population. J Invest Dermatol. 2018;138(8):1874–7.
20. Ujiie H, Muramatsu K, Mushiroda T, Ozeki T, Miyoshi H, Iwata H, Nakamura A, Nomoto H, Cho KY, Sato N, Nishimura M. HLA-DQB1* 03: 01 as a biomarker for genetic susceptibility to bullous pemphigoid induced by DPP-4 inhibitors. J Invest Dermatol. 2018;138(5):1201–4.
21. Esmaili N, Mortazavi H, Chams-Davatchi S, Daneshpazhoooh M, Rayati Damavandi M, Aryanian Z, Amirzargar AA. Association between HLA-DQB1* 03: 01 and Bullous pemphigoid in Iranian patients. Iran J Immunol. 2013;10(1):1–9.
22. Gao XH, Winsey S, Li G, Barnardo M, Zhu XJ, Chen HD, Song F, Zhai N, Fuggle S, Wojnarowska F. HLA-DR and DQ polymorphisms in bullous pemphigoid from northern China. Clin Exp Dermatol. 2002;27(4):319–21.
23. Okazaki A, Miyagawa S, Yamashina Y, Kitamura W, Shirai T. Polymorphisms of HLA-DR and-DQ genes in Japanese patients with bullous pemphigoid. J Dermatol. 2000;27(3):149–56.
24. Delgado JC, Turbay D, Yunis EJ, Yunis JJ, Morton ED, Bhol K, Norman R, Alper CA, Good RA, Ahmed R. A common major histocompatibility complex class II allele HLA-DQB1* 0301 is present in clinical variants of pemphigoid. Proc Natl Acad Sci U S A. 1996;93(16):8569–71.
25. Chagury AA, Sennes LU, Gil JM, Kalil J, Rodrigues H, Rosales CB, Miziara ID. HLA-C* 17, DQB1* 03: 01, DQA1* 01: 03 and DQA1* 05: 05 alleles associated to bullous pemphigoid in Brazilian population. Ann Dermatol. 2018;30(1):8–12.
26. Vuong TB, Do DM, Ong PT, Le TV. HLA-DRB1 and DQB1 genetic susceptibility to pemphigus vulgaris and pemphigus foliaceus in Vietnamese patients. Dermatol Reports. 2022;14(2):9286.
27. Lombardi ML, Mercuro O, Pirozzi G, Manzo C, Lombari V, Ruocco V, Schiavo AL, Guerrera V. Common human leukocyte antigen alleles in pemphigus vulgaris and pemphigus foliaceus Italian patients. Journal of investigative dermatology. 1999;113(1):107–10.
28. Zivanovic D, Bojic S, Medenica L, Andric Z, Popadic D. Human leukocyte antigen class II (DRB1 and DQB1) alleles and haplotypes frequencies in patients with pemphigus vulgaris among the Serbian population. HLA. 2016;87(5):367–74.
29. Párnická Z, Švecová D, Javor J, Shawkatová I, Buc M. High susceptibility to pemphigus vulgaris due to HLA-DRB1* 14: 54 in the Slovak population. Int J Immunogenet. 2013;40(6):471–5.
30. Sáenz-Cantele AM, Fernández-Mestre M, Montagnani S, Calebotta A, Balbas O, Layrisse Z. HLA-DRB1* 0402 haplotypes without DQB1* 0302 in Venezuelan patients with pemphigus vulgaris. Tissue Antigens. 2007;69(4):318–25.
31. Gil JM, Weber R, Rosales CB, Rodrigues H, Sennes LU, Kalil J, Chagury A, Miziara ID. Study of the association between human leukocyte antigens (HLA) and pemphigus vulgaris in Brazilian patients. Int J Dermatol. 2017;56(5):557–62.
32. Li S, Zhang Q, Wang P, Li J, Ni J, Wu J, Liang Y, Leng RX, Pan HF, Ye DQ. Association between HLA-DQB1 polymorphisms and pemphigus vulgaris: A meta-analysis. Immunol Invest. 2018;47(1):101–12.
33. Zivadinov, R., Uxa, L., Bratina, A., Bosco, A., Srinivasaraghavan, B., Minagar, A., Ukmar, M., yen Benedetto, S. and Zorzon, M., 2007. HLA-DRB1* 1501,-DQB1* 0301,-DQB1* 0302,-DQB1* 0602, and-DQB1* 0603 alleles are associated with more severe disease outcome on MRI in patients with multiple sclerosis. Int Rev Neurobiol. 2007;79:521–35.
34. Gorodezky C, Alaez C, Murguía A, Rodríguez A, Balladares S, Vazquez M, Flores H, Robles C. HLA and autoimmune diseases: Type 1 diabetes (T1D) as an example. Autoimmun Rev. 2006;5(3):187–94.
35. Kahaly GJ, Frommer L, Schuppan D. Celiac disease and endocrine autoimmunity–the genetic link. Autoimmun Rev. 2018;17(12):1169–75.
36. Lee JE, Reveille JD, Ross MI, Platsoucas CD. HLA-DQB1* 0301 association with increased cutaneous melanoma risk. Int J Cancer. 1994;59(4):510–3.
37. Khalil I, Lepage V, Douay C, Morin L, Al-Daccak R, Wallach D, Binet O, Lemarchand F, Degos L, Hors J. HLA DQB1* 0301 allele is involved in the susceptibility to erythema multiforme. J Invest Dermatol. 1991;97(4):697–700.
38. Chan LS, Hammerberg C, Cooper KD. Significantly increased occurrence of HLA-DQB1* 0301 allele in patients with ocular cicatricial pemphigoid. J Invest Dermatol. 1997;108(2):129–32.
39. Setterfield J, Theron J, Vaughan RW, Welsh KI, Mallon E, Wojnarowska F, Challacombe SJ, Black MM. Mucous membrane pemphigoid: HLA-DQB1* 0301 is associated with all clinical sites of involvement and may be linked to antibasement membrane IgG production. Br J Dermatol. 2001;145(3):406–14.
40. Wu MS, Hsieh RP, Huang SP, Chang YT, Lin MT, Chang MC, Shun CT, Sheu JC, Lin JT. Association of HLA-DQB1*0301 and HLA-DQB1*0602 with Different Subtypes of Gastric Cancer in Taiwan. Jpn J Cancer Res. 2002;93(4):404–10.
41. Duarte-Rey C, Pardo AL, Rodríguez-Velosa Y, Mantilla RD, Anaya JM, Rojas-Villarraga A. HLA class II association with autoimmune hepatitis in Latin America: a meta-analysis. Autoimmun Rev. 2009 Feb 1;8(4):325–31.
Dylan Thibaut, 1 Fourth-Year Medical Student. Lake Erie College of Osteopathic Medicine, Bradenton, United States.
Ryan Witcher, 1 Fourth-Year Medical Student. Lake Erie College of Osteopathic Medicine, Bradenton, United States.
Breana Barnes, 1 Fourth-Year Medical Student. Lake Erie College of Osteopathic Medicine, Bradenton, United States.
Kersten T. Schroeder, 2 PhD. University of Central Florida, Orlando, United States.
About the Author: Dylan Thibaut is a fourth-year medical student of a four-year program to become a physician. He was the president of the student research association at Lake Erie College of Osteopathic Medicine in Bradenton.
Correspondence: Kersten T. Schroeder. Address: 4000 Central Florida Blvd, Orlando, FL 32816, United States. Email: Kersten.Schroeder@ucf.edu
Editor: Francisco J. Bonilla-Escobar; Student Editors: Ahmed Nahian & Bahadar Srichawla; Proofreader: Amy Phelan; Layout Editor: Ana Maria Morales; Process: Peer-reviewed
Cite as Thibaut D, Witcher R, Barnes B, Schroeder KT. HLA-DQB1*0301 in Bullous Pemphigoid and Pemphigus Vulgaris: A Meta-Analysis. Int J Med Stud. 2023 Jul-Sep;11(3):199-205.
Copyright © 2023 Dylan Thibaut, Ryan Witcher, Breana Barnes, Kersten T. Schroeder
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 11, NUMBER 3, September 2023