Review
Establishing a Causal Link between Ankylosing Spondylitis and Inflammatory Bowel Disease:
A Review of the Literature
Jaclyn Rivington1, Michael Gillett1
doi: http://dx.doi.org/10.5195/ijms.2016.152
Volume 4, Number 2: 55-63
Received 07 10 2015:
Accepted 17 07 2016
ABSTRACT
The link between ankylosing spondylitis and inflammatory bowel disease is unclear,
however it is hypothesized that there is a causal link between the inheritance of
a human leukocyte antigen B27 allele and the development of inflammatory bowel disease
symptoms in ankylosing spondylitis patients. Research articles assessing the relationship
between ankylosing spondylitis, inflammatory bowel disease and the human leukocyte
antigen B27 antigen were collected from the PubMed database. Patients expressing the
human leukocyte antigen B27 allele have a demonstrated predisposition to developing
symptoms of inflammatory bowel disease and sacroiliitis in ankylosing spondylitis.
However, human leukocyte antigen B27 is considered to be just a contributing factor
in the disease, as interleukin-23, natural killer cells, and alterations to the microbiome
have also demonstrated an active role in the development of symptoms. More longitudinal
studies using larger cohorts are needed to further substantiate a direct causal relationship
between ankylosing spondylitis and inflammatory bowel disease.
Keywords:
HLA-B27 Antigen;
Spondylitis;
Ankylosing;
Inflammatory Bowel Diseases;
Sacroiliitis;
Killer Cells;
Natural.
Introduction
Ankylosing spondylitis (AS) is a debilitating disease found in 0.5% of the population.1 It is commonly diagnosed in young Caucasian men aged 20-40 and is characterized by
sacroiliitis and fusion of vertebrae. AS is classified as a seronegative arthropathy,
which are arthropathies that involve the axial skeleton and are negative for rheumatoid
factor. Other arthropathies include: psoriatic arthritis, reactive arthritis, and
enteropathic arthritis. Diagnosis of AS is commonly delayed by 5–6 years due to the
lack of specific biomarkers and variation in clinical presentation.2 Better understanding of the candidate genes and pathogenesis of this incapacitating
disease will hopefully result in earlier diagnosis and more targeted treatment.
AS patients most commonly present with uveitis, aortitis or inflammatory bowel disease
(IBD) in addition to classic symptoms of chronic lower back pain. Development of uveitis
in AS patients appears to be correlated with disease duration, the theory being that
chronic inflammation and immune disruption in AS leaves the patient more vulnerable
to developing uveitis.3 However, the co-presentation of IBD and AS appears to be less chronological.
Only 5–10 % of AS patients present with overt IBD; conversely upwards of 60% of AS
patients have subclinical gut inflammation when investigated through more invasive
means such as ileocolonoscopy.4-6 One study found that nearly all patients with regional enteritis, along with the
human leukocyte antigen (HLA-B27) allele, were prone to developing AS.7 Numerous studies have further demonstrated the close relation between IBD and AS.8,9 Both of these diseases are currently posited as being autoimmune in etiology, and
share some similar genes of interest such as IL-23.10 Furthermore, signs of gut inflammation are seen prior to the diagnosis of AS and
thus may play a role in the etiologic pathway.3
AS has a strong genetic link.11,12 More than 90% of AS patients have the HLA-B27 allele, but its role in the underlying
etiology remains unclear. There appears to be an interplay between not only the HLA-B27
genetic predisposition, but other genes, environmental and immunological factors.
These will be outlined below and in Figure 1.
Figure 1.
Major Contributors to the Development of Ankylosing Spondylitis
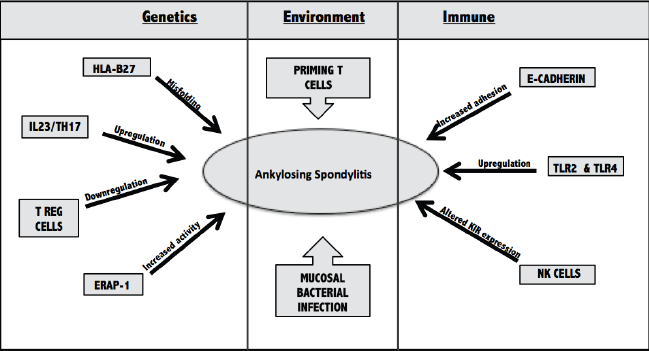
HLAB27=human leukocyte antigen B27; IL23=interleukin 23; Th17=T helper cell 17; T
reg cell=T regulatory cell; TLR2=Toll-like Receptor 2; KIR=Killing Inhibitory Receptor;
NK=Natural Killer cells.
Genetics
HLA-B27 is a major histocompatibility complex (MHC) class 1 antigen that has an established
link with AS. It plays a role in presenting self-antigens to T cells. Research suggests
that misfolding of the protein encoded by this allele leads to its accumulation in
the endoplasmic reticulum (ER).4 This intra-cellular accumulation may then precipitate an inflammatory response seen
both in the joints and in extra-articular sites of AS patients.
Recent Genome Wide Associated Studies (GWAS) have discovered two prospective pathways
in the etiology of AS—interleukin 23/T-helper 17 cell (IL23/Th17) and endoplasmic
reticulum aminopeptidase-1 (ERAP-1). IL23 is produced by antigen presenting cells
and is responsible for upregulating Th17 cells.13 Th17 cells produce pro-inflammatory cytokines (IL17, IL22, TNF-alpha) and are linked
to the development of autoimmune diseases.14 Higher levels of Th17 cytokines have been measured in AS patients, and anti-TNF-alpha
therapy has been shown to downregulate this cell population and provides beneficial
therapy for AS patients.14,15 An upregulation in the Th17 pathway also results in a reciprocal down-regulation
in the regulatory T cell (Treg) pathway, which normally protects against autoimmune
disease.16 The level of evidence supporting the role of the Th17 pathway in AS pathogenesis
continues to increase—there have been similar findings in animal models and most recently
with therapeutic monoclonal antibodies.17,18
The second pathway of interest is endoplasmic reticulum aminopeptidase 1 (ERAP-1),
an aminopeptidase responsible for cleaving peptides in the ER before they are loaded
into the MHC1. Recent observation demonstrates that ERAP-1 alterations only influence
AS patients that are HLA-B27 positive.19 Patients with higher aminopeptidase activity have altered expression of free heavy
chains (FHC) on the surface of their circulating monocytes. FHC’s are commonly referred
to as class one molecules without the B2 microglobulin.20 These FHC dimers are recognized on the surface by inhibitory killing immunoglobulin
receptors (KIR) of NK cells, and will be further discussed in the Immune section.21
Environment
Alterations in gut microbiota is suspected of playing a role in gut inflammation in
AS pathogenesis. Previous studies have shown molecular similarities between HLA-B27
and Klebsiella pneumoniae.22 Klebsiella and anti-Klebsiella antibodies have been isolated from the bowels of patients with active AS and parallel
exacerbations were seen between Klebsiella levels and IBD symptoms.22-24
It is hypothesized that long-term inflammation of the intestinal mucosa caused by
bacterial infections could eventually catalyze an inflammatory response within the
joint. However, live bacteria have never been isolated from the synovium and chronic
anti-bacterial treatment has been futile at slowing disease progression. A recent
study suggests that, more conceivably, T cells are primed in the gut and then migrate
to the synovium.24 Once in the synovium, cluster differentiation 163 (CD163) macrophages are activated
and release a very specific cytokine profile, including high TNF-alpha and low IL10.25
Immune system
AS patients have shown increased permeability in the mucosal lining of their gastrointestinal
tract, which is considered a first line, innate immune defense.26 E-cadherin, an epithelial junctional protein, may contribute to this mucosal disturbance.
The down-regulation of E-cadherin is commonly associated with cancer metastasis, however,
there has been recent proof of higher levels of this adhesion complex in patients
with IBD and spondyloarthropathies.27
Alteration in the expression of Toll-like receptors (TLR4 and TLR2) in the gut mucosa
as well as the synovium of patients with AS could indicate a link between the innate
immune system and presentation of IBD in AS.28,29 TLR’s are a family of membrane proteins that, when bound by a pathogen associated
molecular pattern (PAMP), activate a pathway that mediates inflammation. This pathway
is essential in the initiation of host defense. After TNF-alpha inhibitor therapy,
down-regulation of TLR-4 and remittance of symptoms was observed which suggests a
prominent role of this protein in AS and potential contribution to the etiology.28
A final immune regulatory mechanism that has been suggested is the alteration in natural
killer (NK) cell defense. Killer immunoglobulin-like receptors (KIR) are found predominantly
on NK cells, T cells and minor subsets of CD4 and CD8 cells. They act by binding to
MHC class 1 and altering the cytokine profile to promote survival of the cell.30 KIR’s are unique as they have the ability to recognize different HLA-class one allotypes,
which could be linked to the AS association with HLA-B27. Haroon et al, 2012, demonstrated
an increased risk associated with KIR3DL2 and a protective effect of KIRDL1.31 To date, KIRDL1 is the only KIR known to recognize and bind to the HLA-B alleles.32 Therefore, alterations to KIR expression could alter innate immune mechanisms of
defense, predisposing patients to development of AS.
Current literature reveals a consistent genetic link between AS and HLA-B27, however
further research has demonstrated that this is a very complex, multifactorial and
oligogenic disease. The association of certain genes with disease is a first step
in a full understanding of etiology, and current research aims to understand the sequence
of pathogenesis to develop more targeted therapy. This paper will further explore
the relationship of HLA-B27 with the extra-articular symptoms of IBD in AS. It is
hypothesized that there is a causal link between the inheritance of an HLA-B27 allele
and the development of IBD symptoms in AS patients.
Search Strategy and Selection Criteria
The PubMed database (01 January 2004 – 30 December 2014) was searched using the terms:
“Ankylosing Spondylitis”, “Inflammatory Bowel Disease” and “HLA-B27 Antigen”. These
terms were searched both as basic terms and “MeSH” terms. The author examined the
titles and abstracts for articles that included: (1) Both IBD and Ankylosing Spondylitis
in the abstract; (2) Human subjects, (3) Published in English, (4) Full reports (not
just abstracts) and (5) Addressed the link and/or etiology of IBD and AS. Articles
that did not meet these criteria were excluded. Full text articles were obtained and
classified according to their study type. Articles included in the review can be found
in Table 1. Studies were classified using the evidence table created by the authors, as shown
in Table 2. The methodology used for reviewing articles for inclusion is outlined in Figure 2.
Table 1.
Studies Evaluating the Genetics of Ankylosing Spondylitis
| Study |
Study Design |
Level of Evidence |
Study Population |
Therapy or Exposure |
Outcomes and Results |
| Ciccia et al (2014) |
Case control with controls |
3 |
30 AS patients (human leukocyte antigen (HLA-B27+)); 15 Crohn’s disease (CD) patients
& 10 normal subjects
|
Consecutive gut biopsies were analyzed for evidence of misfolding of HLA-B27. Unfolded
protein response (UPR) and autophagy were assessed via RT-PCR and IHC. This was then
compared to the regulation of IL-23 expression on lamina propria mononuclear cells
(LPMCs).
|
Upregulation of autophagy genes were observed in AS and CD patients. Autophagy was
also required to modulate expression of LPMCs. This suggests that misfolding of HLA-B27
occurs in the gut of AS patients and in accompanied by the activation of autophagy.
Autophagy (not UPR) is associated with the increased expression of IL-23 in AS patients.
|
| Duarte et al (2014) |
Prospective Cohort |
3 |
1424 patients; 235 with disease onset before 16 years old |
Analyze the characteristics of juvenile-onset spondyloarthritis (SpA) (<16 yr) with
a group of adult-onset SpA patients.
|
Juvenile-Onset was characterized by male gender, peripheral involvement (arthritis),
positive HLA-B27 vs adult-onset was associated with inflammatory bowel disease, dactylitis
and nail involvement.
|
| Wei et al (2013) |
Case control |
3 |
475 AS patients; 475 healthy subjects in a Taiwanese population |
Investigating loci other than endoplasmic reticulum aminopeptidase-1 (ERAP-1) and
HLA-B27 for AS susceptibility. A recent GWAS showed two potential loci 5q14.3 and
12q12.
|
The two markers showed no association between polymorphisms and AS susceptibility,
however significant association was seen between 12q12 and inflammatory bowel disease.
|
| Matzkies et al (2012) |
Case control |
3 |
39 AS patients; 42 controls. Average age 47, average age duration was 22 years |
Sera were tested by ELISA for IBD-associated serologies (ANCA, anti-Cerevisiae Ab
IgG & IgA, Anti-I2, Anti-OmpC and anti-CBir1). BASDAI was also completed for AS patients.
|
Cal protectin levels were elevated in 41% of AS patients. fCAL-positive AS patients
displayed higher IBD antibodies. Further studies needed to determine whether fCAL
can be used to identify a subgroup of AS patients with IBD.
|
| Skare et al (2012) |
Cross sectional |
4 |
1318 SpA patients; 65% white; 31.3% Brazilian; 3.7% mixed. 65.1% with AS; 18.3% psoriatic
arthritis, 6.8% undifferentiated
|
Did an array of tests and looked at other variables to determine whether ethnicity
plays a role in the progression of spondyloarthropathies.
|
Gender, family history, presence of peripheral arthritis and IBD were consistent across
all three groups. Brazilians had higher MASE score and worse performance on the quality
of life questionnaire.
|
| D’Inca et al (2009) |
Case control with no control |
4 |
142 UC patients; 120 CD patients |
Looked at the prevalence of articular pain in IBD patients. |
Prevalence of self-reported articular symptoms exceeds 40%, with 9.5% incidence in
1 year follow up.
|
| Togrol et al (2009) |
Case control |
3 |
30 AS patients; 19 age and sex matched controls |
Analyzed HLA-B27, Anti-gliadin (AGA), & endomysial antibodies (EMA). AGA positive
patients were examined by a gastroduodenoscope.
|
Eleven AS patients were AGA positive (0 controls); 3 were also EMA positive. Destruction
of villi was seen the in three AGA/EMA positive patients. AGA positivity might contribute
to pathogenesis of AS (increasing intestinal permeability), more research is required.
|
| Orchard et al (2008) |
Cross sectional |
4 |
44 CD patients |
Clinical evaluation of symptom questionnaire, rheumatological exam, HLA genotyping,
MRI of sacroiliac joints.
|
40% of patients had evidence of sacroiliitis, 5 fulfilled the criteria for AS. No
association of HLA with sacroilitis, but definitely with AS. Possession of HLA-B27
conveys an increased risk of developing axial inflammation in CD.
|
| Aydin et al (2008) |
Case control |
3 |
175 patients with AS; 47 with undifferentiated SpA; 103 healthy controls |
Questioned for demographic features, BASDAI scores, BASRI and mSASSS scores were acquired;
Anti-Sacchoromyces Cervesiae Antibodies (ASCA) was measured with ELISA.
|
Increased prevalence of ASCA in AS and undifferentiated SpA. ASCA can also be a marker
for radiological damage and more severe course AS.
|
| Turkcapar et al (2006) |
Case control with no control |
4 |
162 with IBD |
Complete rheumatological examination, evaluated via the ESSG criteria for SpA. Analyzed
HLAB27, B51 & ANCA.
|
Prevalence of SpA and AS in IBD – 45.7% & 9.9%. High prevalence of asymptomatic sacroiliitis
in IBD, an early diagnosis of inflammatory arthritis could prevent a disability due
to SpA and AS.
|
BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; BASRI=Bath Ankylosing Spondylitis
Radiology Index; ELISA=enzyme-linked immunosorbent assay; ESSG=European Spondyloarthropathy
Studies Group; GWAS=genome-wide association study; IHC=immunohistochemistry; MASE=Maastricht
Ankylosing Spondylitis Enthesitis Score; MRI=magnetic resonance imaging; mSASSS=modified
Stoke Ankylosing Spondylitis Spinal Score; RT-PCR=reverse transcription polymerase
chain reaction.
Table 2.
Criteria Used to Evaluate Study Quality
| 0 |
Preclinical studies–including experimental studies and animal models |
| 1 |
Randomized controlled trials |
| 2 |
Non-randomized controlled trial – a prospective (pre-planned) study with a predetermined
eligibility criteria and outcome measures
|
| 3 |
Observational studies with controls-includes retrospective, case-control studies,
and cohort studies
|
| 4 |
Observational studies without controls – includes cohort studies without controls,
case series without controls, case studies without control
|
Figure 2.
Flow Diagram for Article Selection
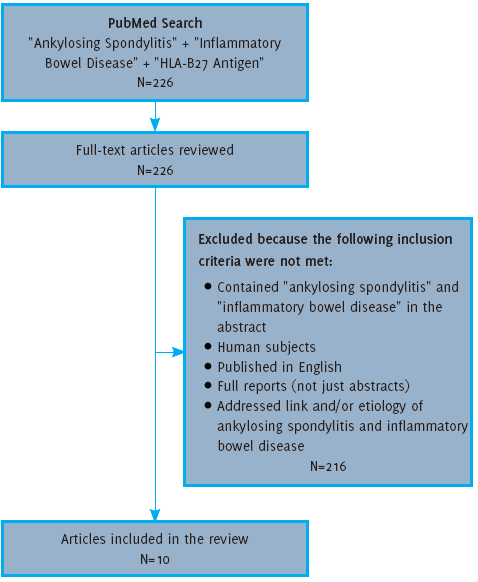
Search Results
Using the search criteria as outlined in the methods, the literature search provided
226 articles, all of which were reviewed. Based on the exclusion criteria, 10 of these
articles were included for the purposes of this review. These 10 articles included
six level-three case-control studies and four level-four cohort studies without controls.
A summary of candidate genes and biomarkers which will be outlined in this review
is available in Table 3.
Table 3.
Candidate Genes and Biomarkers Outlined in This Review
| Candidate Genes/Biomarkers |
Relevance |
Future direction |
| HLA-B27 |
90% link to AS. Current understanding: Misfolding of B2 leads to intracellular accumulation
→ stress/inflammatory response
|
More research needed to understand the exact mechanism |
| IL23/Th17 |
Upregulation of Th17 cells via IL23 → development of autoimmune conditions |
Considered to be a gene of current major research interest. Overlap seen between AS
and IBD pathogenesis. May indicate autoimmune mechanism
|
| ERAP-1 |
Aids in cleaving peptides for MHC1 loading |
Second strongest association with AS. Requires further investigation of link with
NK cells
|
| ASCA |
Currently used in cystic fibrosis and liver cirrhosis |
Extensive variability in the literature. More sensitive and specific assays required |
| fCal |
Higher titers found in patients with IBD symptoms |
Inconclusive. Consider conducting case control studies with patients on TNF inhibitor
& NSAID therapy
|
| AGA & anti-EMA |
Currently used in celiac disease |
Postulated as increasing intestinal permeability. Further research is required |
HLAB27=human leukocyte antigen B27; Th17=T helper 17 cells; IL23=interleukin-23; ERAP-1=endoplasmic
reticulum aminopeptidase-1; ASCA=anti-Sacchoromyces cervesiae antibodies; EMA=anti-endomysial antibodies; AGA=anti-gliadin antibodies; fCal=fecal
calprotectin.
Genetics
Ciccia et al (2014) investigated the mechanism of IL23 expression in a study of 30
HLA-B27 positive AS patients, 15 Crohn’s Disease (CD) and 10 normal subjects.13 Sequential gut biopsies were taken from the subjects, then autophagy and unfolded
protein response (UPR) were evaluated in mononuclear cells of the lamina propria by reverse transcription polymerase chain reaction. The researchers noted upregulation
of protein expression in the autophagy pathway via LC311, ATG5 and ATG12 (r2=0.66, 0.68, 0.77 respectively, p<0.0001). However, no significant expression of UPR
genes, namely HSPA5, was observed when compared to controls (p<0.0001). This research
suggested that misfolding of HLA-B27 in the gut mucosa and modulation of IL23 is associated
with autophagy rather than UPR.
Potential IBD & AS Biomarkers
Anti-Sacchoromyces cervesiae Antibodies (ASCA)
Aydin et al measured anti-Sacchoromyces cervesiae antibodies, a characteristic marker of CD, and Bath Ankylosing Spondylitis Disease
Activity Index (BASDAI), a common self assessment used in AS diagnosis, in 175 AS
patients, 47 undifferentiated spondyloarthropathy (uSpA) patients, an incomplete or
early form of a definitive seronegative spondyloarthropathy, and 103 healthy controls.6 There was an increased prevalence of ASCA in patients with AS and uSpA (p<0.0008,
p<0.02 respectively), however no correlation was seen between ASCA and erythrocyte
sedimentation rate (ESR), C-Reactive Protein (CRP) and BASDAI scores (p=0.75, 0.79,
0.28 respectively). ASCA-positive patients also required anti TNF-alpha therapy more
often (p<0.006). This study failed to show any correlation with ASCA and HLA-B27 (p=1).
Fecal Calprotectin
Matzkies et al conducted a pilot study to measure fecal calprotectin (fCal), a biochemical
marker for intestinal wall inflammation, and other IBD-related serology (ASCA, ANCA,
anti-OmpC, anti-CBir1 and anti-I2) in 39 AS patients and 42 controls.33 AS patients were mostly male and 76% were HLA positive. The fCal levels were significantly
higher in the AS group (p<0.0002), as were ANCA, ASCA, and anti-CBir1 (p<0.001, 0.023,
0.003 respectively). The fCal-positive AS patients also demonstrated higher titers
of IBD antibodies, namely IgA ASCA, IgG ASCA and CBir1 (p<0.05, 0.003, 0.002 respectively)
and higher BASDAI scores (p=0.016). However, no correlation was shown between the
presence of the HLA allele and high fCal levels (p=0.71).
Anti-endomysial (EMA) and Anti-gliadin (AGA) Antibodies
Togrol et al analyzed 30 AS patients and 19 controls with basic clinical serology
for HLA-B27, AGA and EMA, both IgG and IgA antibody subtypes.34 Radiological assessment was also performed on all subjects. Gastroduodenoscopy was
only performed on AGA-positive patients. Only 11 of the 30 AS patients were positive
for AGA (p<0.001), and three of 30 were positive for EMA, while none of the controls
were positive for either antibody. No correlation was found between presence of AGA
and severity of AS.
Relevance of Ethnicity
Skare et al evaluated 1318 consecutive patients with spondyloarthropathies (SpA),
in 29 different referral centers throughout Brazil.35 They compared and contrasted the disease expressivity and variance of SpA among different
ethnic backgrounds. This was achieved through a standard protocol of investigation
outlined by the researchers, whereby information was retrieved from patient interviews
and multiple self-assessment scores (BASDAI, BASFI, AS quality of life questionnaire
etc.). The physical exam was guided through many standardized scores and activity
indices, such as the Bath Ankylo-sing Spondylitis Metrology Index (BASMI) and the
Maastricht Ankylosing Spondylitis Enthesitis Score (MASES). In the cohort, 65% of
patients were white (compared to the general population of 48.7%), 31% were African-Brazilian
and 3.7% were of other ethnicities. White patients presented with more psoriasis and
linkage to HLA-B27 (p=0.002, 0.014 respectively). African-Brazilian patients reported
worse back pain and hip involvement (p=0.041, 0.020 respectively) in addition to a
lower patient global assessment and a lower quality of life (p=0.011, <0.0001 respectively).
HLA-negative patients were characterized as having no family history, later disease
onset and limited extra-articular manifestations. However, no statistical data was
provided for these statements.
A second ethnic study, conducted by Wei et al (2013) investigated genes unveiled in
the European based genome-wide association study of AS, specifically EDIL3, HAPLN1
and ANO6 at 12q12 and their association to AS.36 The study included 475 AS patients and 475 controls, 90.7% of the AS patients were
HLA-B27-positive. They used genetic markers for the relevant genes, along with multiple
AS activity indices and relevant physical exam findings to compare patients. In the
Taiwanese population, EDIL3, HAPLN1 and ANO6 demonstrated no link to AS (p=0.3033,
0.6754, 0.5266 respectively), however there was a significant association found between
ANO6 and IBD (p=0.0131).
IBD Patients with Joint Manifestations
Turkcapar et al evaluated 162 adult patients with either Crohn’s disease (CD) or ulcerative
colitis (UC) for spondyloarthropathy (SpA) by a full rheumatological assessment and
radiography.2 HLA-B27, B51 and anti-neutrophil cytoplasmic antigen (ANCA) were also tested in all
patients. The prevalence of IBD and SpA was 45.7%. They found that the HLA-B27 allele
was more common in the patients with sacroiliitis, peripheral arthritis and enthesitis
(p<0.001 for all). Additionally, 13.6% of patients exhibited no musculoskeletal manifestations
on physical examination, but were determined to have grade-2 sacroiliitis on computer
tomography (CT) and radiographs.
Another study by Orchard et al (2009) investigated 44 patients with CD in a prospective
study.7 They evaluated the patients’ presenting symptoms, rheumatology profile and HLA status
and conducted a radiologic evaluation of the patients’ sacroiliac joints. The researchers
found that 17 of the 44 patients had evidence of sacroiliitis, HLA-B27 was present
in seven of 44 patients (15.9%), all of whom had sacroiliitis and five of whom had
AS (p<0.001). Sacroiliitis appeared to be a common extra-intestinal manifestation
of CD, but HLA-B27 was not associated with isolated sacroiliitis (p=0.057). Conversely,
HLA-B27 could be considered an important risk factor for developing axial SpA in CD,
as 100% of patients with this allele in this study developed sacroiliitis.
In a final IBD study, D’Inca et al surveyed 651 patients over a 12-month period to
determine which extra-intestinal symptoms were apparent in IBD patients.37 Articular pain was reported most frequently, totaling 262 patients, specifically
142 patients with UC and 120 patients with CD. These 262 patients were further evaluated
by rheumatologists for HLA type, degree of joint involvement (axial or peripheral)
and followed up 12 months after the initial survey. HLA-B27 was present in five of
the participants, three of whom had AS. The frequency of HLA-B27 was significantly
higher in the subgroup of patients with axial arthropathy compared to peripheral arthropathy
(OR = 13.1, p<0.00001). Arthropathies were axial in 53%, and polyarticular in 23%
of patients. Of the 40.2% of patients who reported arthralgia in the original survey,
only 9.5% reported symptoms during the 12-month follow up.
Discussion
In this review, the relationship between IBD and AS was examined. There is a significant
overlap between these two diseases, with IBD being the most common extra-articular
symptom in AS and arthralgia being the most common extra-intestinal symptom in IBD.
It nevertheless remains questionable whether there is a causal relationship between
IBD and AS. It is apparent that the inheritance of HLA-B27 does not definitively determine
whether a person will develop AS, as only 1-2% of the population that is HLA-B27 positive
ends up developing AS. There is, however, a more significant link between the presence
of the HLA-B27 allele and a concurrent diagnosis of IBD. Studies show that the majority
of patients with IBD and the HLA-B27 allele will also present with sacroiliitis.7 They suggest that gut inflammation in combination with HLA-B27 almost guarantees
a subsequent diagnosis of AS.
Many of the studies included in this review used a cross-sectional design, which limits
the interpretation of causal relationships in AS.35,36 It allows the researchers to note associations but denies the ability to sequence
events in disease etiology. A Brazilian study juxtaposes epidemiologic variables between
two age groups: juvenile (younger than 16 years of age) and adult-onset (greater than
16 years of age) spondyloarthropathy. Findings indicate that male gender and HLA-B27
are more common in younger patients, whereas IBD and cutaneous psoriasis are more
common in adult-onset SpA. One could extrapolate from this study that IBD is a latter
manifestation of SpA, however this is a cross-sectional design.37 Further longitudinal investigations that evaluate associations between genetic and
environmental risk factors would contribute immensely to understanding the complete
pathogenesis.
The advantage of cross-sectional studies is that researchers can reach a more diverse
population and may also expose associations that serve as a starting point for other,
more focused studies. This was observed in the two studies that analyzed the effect
of ethnicity on the clinical presentation of AS. One unveiled the difference in candidate
gene expression seen in European versus Taiwanese populations, whereas the other highlighted
the variation in symptoms among different ethnicities in Brazil.35,36 The results of these studies suggest that the pathogenesis for AS may be unique in
each ethnic group and that GWAS conducted in one population is not transferable to
another. Further evidence of this was demonstrated by one researcher who noted that
the HLA-B27 allele was found in 80% of white patients compared to 60% of black patients.35 Countries with a mixed demographic may need to take this into consideration when
conducting studies or determining treatment options for patients from different ethnic
backgrounds.
Many studies included in this review involved a sample population that was identified
based on a recent visit to the rheumatologist.7,33,34,36 However, many developing nations do not have equal access to health care, therefore,
patients involved in these studies may be more representative of urban or higher social
class.35 It is, therefore, critical that adequate representation from all social demographics
be included in studies, to gain the best possible understanding of disease manifestation
and treatment protocol.
Another shortcoming of many studies is the small sample size and concomitant lack
of statistical power. Determinants of statistical power include sample size, statistical
significance criteria (p-values) and magnitude of effect. One study referenced an
odds ratio of 13.1 and p<0.00001 with regard to the HLA-B27 allele and the symptoms
of axial arthropathy.38 Both of these values exhibit undeniable statistical significance. However, this result
was based on a mere five patients in the study with HLA-B27. Despite the statistical
significance of this study, the small sample size raises questions about the applicability
of the results to the broader population. Similarly, other studies had sample groups
with fewer than 40 participants.33,34 Larger studies are required to determine concrete relationships.
Multiple studies have investigated the use of selective biomarkers in AS patients.6,33,34 The diagnosis of AS is currently made through a combination of physical examination,
history and radiography, however, due to the mixed clinical presentation and delayed
evidence on radiography, AS is commonly mis-diagnosed or delayed in diagnosis by 5-6
years.2 Accurate and early diagnosis of the disease would allow a treatment regime designed
to delay fusion of vertebrae, stiffening of joints and debilitating effects of AS.
Biomarkers would allow for more precise direction with prognosis and management.39
The anti-ASCA antibody showed higher titers in patients with more symptoms of AS,
but no correlation with the HLA-B27 allele. Anti-ASCA is also used in the evaluation
of patients with liver cirrhosis, cystic fibrosis and Behcet’s disease.6 There appears to be extensive variability in the literature as some studies demonstrated
no correlation between anti-ASCA and SpA, others limit the association to IgA not
IgG, and some claim it is seen in AS but not uSpA.404142 The discrepancy of results may be due to the variation in sensitivity and specificity
in assays. More consistent and reproducible results need to be established before
anti-ASCA could be considered a valid biomarker for AS.
Another biomarker, fCal, showed higher titers in patients with IBD symptoms, but no
relevant association to the HLA allele.33 This study purposely excluded patients that were on TNF-alpha inhibitor or non-steroidal
anti-Inflammatory drugs (NSAID), with the consideration that NSAIDS can increase gut
permeability and that TNF inhibitors may alter gut inflammation.33 Other studies that investigated fCal did not use these exclusion criteria and concluded
that there was no correlation between fCal and gut inflammation, and high levels of
fCal were associated with age, disease duration, raised ESR and serum calprotectin
levels, but not with GI symptoms.43 This exclusion criterion may limit the applicability of results to the general population,
as most patients with AS or underlying gut pathology will likely be on some sort of
therapy. The exclusion of this demographic from the study, therefore, reduces the
relevance and application of the results in the clinical setting. Future studies may
consider evaluating the levels of fCal in both the presence and absence of TNF inhibitors
and NSAIDS to gain a better understanding of how this marker fluctuates with treatment
regimen and flare-ups of IBD.
The final biomarkers uncovered in this review were anti-AGA and anti-EMA, two antibodies
commonly used in celiac disease. Researchers determined that anti-AGA was higher in
the AS group, and also found that three patients who were anti-EMA-positive were also
anti-AGA-positive. This study lacked statistical power as it analyzed only 30 patients.
Its analysis was skewed, as gastroduodenoscopy and mucosal biopsy was only performed
on the 11 patients who were AGA positive, whereas all other negative anti-AGA patients
in the AS group and the entire control group were limited to serological analysis.
Researchers claim that increased inflammatory cell infiltrate was seen in the lamina propria of anti-AGA/EMA-positive patients. However, they did not have a control group for
comparison. Anti-AGA antibodies may be an indicator of AS pathogenesis by increasing
intestinal permeability, but it does not appear to be specific to AS as only 11 of
30 patients had a positive titer. A larger cohort is required to validate these associations.
Current research is heavily focused on understanding the genetic link to AS and how
certain genes play a role in the pathogenesis, but not necessarily on the exact link
between the symptoms of IBD and AS. A limitation to this review is that it is based
on a key word search of the literature, and although IBD and HLA-B27 are brought up
consistently in research relating to AS, it is uncommon to find a directed study on
the relation between the two. However, this is an inherent limitation of the searching
capability of research databases. An example of this can be seen in the three studies
included in this review, all of which targeted a population of subjects with IBD,
but then looked at the prevalence of associated arthralgia.2,7,38 Conversely, the main goal of this paper was to do the opposite: evaluate the likelihood
of AS patients having symptoms of IBD. Given that this kind of study provides the
greatest insights regarding this relationship to date, more directed studies looking
specifically at AS patients need to be undertaken for a clear consensus to be reached.
Future Directions
Pharmaceuticals
Current treatment of AS includes both pharmacological and non-pharmacological approaches.
Pharmacological intervention normally includes NSAIDS, disease modifying anti-rheumatic
drugs (DMARDS) and TNF-alpha inhibitors. Review of TNF-alpha inhibitor therapy has
demonstrated that 40% of AS patients experience an increase in function, reduction
in pain and morning stiffness and overall well-being.44 Further advances in pharmacologic treatment are limited to a full comprehension of
AS pathogenesis, predisposing genes and environmental factors, as this enables manufacturers
to target more disease-specific culprits. As an example, the more conclusive evidence
of IL23R’s role in AS has allowed researchers to manufacture an IL23 specific monoclonal
antibody which is currently undergoing phase two clinical trials.18
Genetics
Studies have confirmed the role of IL23R in AS aetiopathogenesis, and suggested that
this pathway may be a key regulator in the misfolding of the HLA-B27 protein.13 Previous studies suggested that misfolding was due to an unfolded protein response
(UPR) in the cell, however this study proved that it was due to upregulation of autophagy.
Additionally, they localized the misfolding of HLA-B27 to the gut, which further confirms
the suspicion that chronic gut inflammation may be a contributor to the early pathogenesis
of AS.
Other genes such as ERAP were not discussed in this review, as they did not appear
in the search criteria. The development of AS is multifactorial and may even be unique
in each patient, therefore, careful accreditation of all genetic markers unveiled
in the GWAS is necessary in future research.
GIT Therapy
Not all AS patients suffer from IBD, but a large majority have subclinical gut inflammation.
Treatment of this could be a future consideration for clinicians. Multiple studies
have identified bacteria such as Klebsiella, Yersinia, and most recently Faecali-bacterium prausnitzii and Bacteroides fragilis in AS patients.45 A potential route of treatment is fecal microbiota transfer (FMT) – replacement of
missing or overbearing bacteria to the natural multicomponent state. FMT is considered
a highly effective treatment for Clostridium difficile, and was recently accepted by the Food & Drug Administration (FDA) as an experimental
therapy. It has shown variable effectiveness in the treatment of IBD, however, the
success in AS is still under review.46 Furthermore, a pediatric study on IBD concluded that certain micro-biota are more
responsive to TNF therapy and that the levels of certain bacteria could be used as
a predictor of responsiveness to TNF therapy.47
An alternative, less invasive, manipulation of the gut microbiota involves the alteration
of diet through supplementation and probiotics.22 Probiotics improve colitis and IBD symptoms in murine models and human subjects due
to their induction of TLR9 and resulting anti-inflammatory effect.48,49 Adjusting an AS patient’s diet could be used as a complement to conventional pharmaceuticals
in the future.
Radiography
Due to the variation in clinical presentation, AS is a challenging arthropathy to
diagnose. It may be possible to diagnose AS earlier if younger patients with IBD are
screened for signs of spondyloarthropathy.2 A full radiological and rheumatological assessment could drastically reduce the progression
and potential disability of SpA.
Conclusion
This review supplements current evidence that there is a link between IBD and AS.
Many studies prove the predisposition of IBD patients with HLA-B27 to developing symptoms
of sacroiliitis and AS. However, HLA-B27 is considered to be one of many factors in
the disease, not the sole contributor. More longitudinal studies need to be conducted
to clarify whether AS and IBD are concomitant or causal of one another. Similarly,
larger cohorts should be considered to increase the power and relevance of the studies.
The complex pathogenesis, polygenic etiology and variance between ethnicities of AS
has proven to be an obstacle and major focus of research. The discovery of more specific
biomarkers is essential for the early diagnosis of AS and a deeper understanding of
the exact pathogenesis will allow for more directed and effective treatment for this
incapacitating disease.
Acknowledgments
The author would like to thank Saba University School of Medicine for endorsing the
publication of this review article.
Conflict of Interest Statement & Funding
The authors have no funding, financial relationships, or conflicts of interest to
disclose.
Author Contributions
Conceptualization, Critical revision of the manuscript, Approval of the final version:
JR, MG. Data collection, Data analysis and interpretation, Writing: JR. Administrative/technical
advice: MG.
References
1. Golder V, Schachna L. Ankylosing spondylitis: an update. Aust Fam Physician. 2013 Nov;42(11):780–4.
2. Turkcapar N, Toruner M, Soykan I, Aydintug OT, Cetinkaya H, Duzgun N, et al. The prevalence of extraintestinal manifestations and HLA association in patients with
inflammatory bowel disease. Rheumatol Int. 2006 May;26(7):663–8.
3. Tsui FW, Tsui HW, Akram A, Haroon N, Inman RD. The genetic basis of ankylosing spondylitis: new insights into disease pathogenesis. Appl Clin Genet. 2014 May 22;7:105–15.
4. De Vos M. Joint involvement associated with inflammatory bowel disease. Dig Dis. 2009;27(4):511–5.
5. Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2006 Jun;20(3):451–71.
6. Aydin SZ, Atagunduz P, Temel M, Bicakcigil M, Tasan D, Direskeneli H. Anti-Saccharomyces cerevisiae antibodies (ASCA) in spondyloarthropathies: a reassessment. Rheumatology (Oxford). 2008 Feb;47(2):142–4.
7. Orchard TR, Holt H, Bradbury L, Hammersma J, McNally E, Jewell DP, et al. The prevalence, clinical features and association of HLA-B27 in sacroilitis associated
with established Crohn’s Disease. Aliment Pharmacol Ther. 2009 Jan;29(2):193–7.
8. Jacques P, Elewaut D. Joint expedition: linking gut inflammation to arthritis. Mucosal Immunol. 2008 Sep;1(5):364–71.
9. Steer S, Jones H, Hibbert J, Kondeatis E, Vaughan R, Sanderson J, et al. Low back pain, sacroiliitis, and the relationship with HLA-B27 in Crohn’s disease. J Rheumatol. 2003 Mar;30(3):518–22.
10. Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008 Jun;8(6):458–66.
11. Brown MA, Kennedy LG, MacGregor AJ, Darke C, Duncan E, Shatford JL, et al. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the
environment. Arthritis Rheum. 1997 Oct;40(10):1823–8.
12. Jarvinen P. Occurrence of ankylosing spondylitis in a nationwide series of twins. Arthritis Rheum. 1995 Mar;38(3):381–3.
13. Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression
of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2014 Aug;73(8):1566–74.
14. Haroon N. Endoplasmic reticulum aminopeptidase 1 and interleukin-23 receptor in ankylosing spondylitis. Curr Rheumatol Rep. 2012 Oct;14(5):383–9.
15. Davis JC Jr. Understanding the role of tumour necrosis factor inhibition in ankylosing spondylitis. Semin Arthritis Rheum. 2005 Feb;34(4):668–77.
16. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and TH17 cells. Annu Rev Immunol. 2009;27:485–517.
17. Smith JA, Colbert RA. Review: the interleukin-23/interleukin 17 axis in spondyloarthritis pathogenesis:
TH17 and beyond. Arthritis Rheumatol. 2014 Feb;66(2):231–41.
18. Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis:
a randomised, double-blind, placebo-controlled trial. Lancet. 2013 Nov;382(9906):1705–13.
19. Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide
handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011 Jul 10;43(8):761–7.
20. Tsai WC, Chen CJ, Yen JH, Ou TT, Tsai JJ, Liu CS, et al. Free HLA class I heavy chain-carrying monocytes—a potential role in the pathogenesis
of spondyloarthropathies. J Rheumatol. 2002 May;29(5):966–72.
21. Kollnberger S, Chan A, Sun MY, Chen LY, Wright C, di Gleria K, et al. Interaction of HLA-B27 homodimers with KIR3DL1 and KIR3DL2, unlike HLA-B27 heterotrimers,
is independent of the sequence of bound peptide. Eur J Immunol. 2007 May;37(5):1313–22.
22. Ebringer A, Rashid T, Tiwana H, Wilson C. A possible link between Crohn’s disease and ankylosing spondylitis via Klebsiella
infections. Clin Rheumatol. 2007 Mar;26(3):289–97.
23. Ebringer A, Rashid T, Wilson C, Ptaszynska, T, Fielder M. Ankylosing spondylitis, HLA-B27 and Klebsiella – an overview: proposal for early diagnosis
and treatment. Curr Rheumatol Rev. 2006;2(1):55–68.
24. Wilson C, Rashid T, Tiwana H, Beyan H, Hughes L, Bansal S, et al. Cytotoxicity responses to peptide antigens in rheumatoid arthritis and ankylosing
spondylitis. J Rheumatol. 2003 May;30(5):972–8.
25. Faustini F, Zoli A, Ferraccioli GF. Immunologic and genetic links between spondyloarthropathies and inflammatory bowel
diseases. Eur Rev Med Pharmacol Sci. 2009 Mar;13 Suppl 1:1–9.
26. Martínez-González O, Cantero-Hinojosa J, Paule-Sastre P, Gómez-Magán JC, Salvatierra-Ríos D. Intestinal permeability in patients with ankylosing spondylitis and their healthy
relatives. Br J Rheumatol.1994 Jul;33(7):644–7.
27. Demetter P, Baeten D, De Keyse F, De Vos M, Van Damme N, Vebruggen G, et al. Subclinical gut inflammation in spondyloarthropathy patients is associated with upregulation
of the E-cadherin/catenin complex. Ann Rheum Dis. 2000 Mar;59(3):211–6.
28. De Rycke L, Vandooren B, Kuithof E, De Keyser F, Veys EM, Baeten D. Tumour necosis factor alpha blockade treatment down-modulates the increased systemic
and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005 Jul;52(7):2146–58.
29. Yang ZX, Liang Y, Zhu Y, Li C, Zhang LZ, Zeng XM, et al. Increased expression of Toll-like receptor 4 in peripheral blood leucocytes and serum
levels of some cytokines in patients with ankylosing spondylitis. Clin Exp Immunol. 2007 Jul;149(1):48–55.
30. Cauli A, Piga M, Dessole G, Porru G, Floris A, Vacca A, et al. Killer-cell immunoglobulin-like receptors (KIR) and HLA-class I heavy chains in ankylosing
spondylitis. Drug Dev Res. 2014 Nov;75 Suppl 1:S15–9.
31. Haroon N, Tsui FW, Uchanska-Ziegler B, Ziegler A, Inman RD. Endoplasmic reticulum aminopeptidase 1 (ERAP1) exhibits functionally significant interaction
with HLA-B27 and relates to subtype specificity in ankylosing spondylitis. Ann Rheum Dis. 2012 Apr;71(4):589–95.
32. Lopez-Larrea C, Blanco-Gelaz MA, Torre-Alonso JC, Bruges Armas J, Suarez-Alvarez B, Pruneda L, et al. Contribution of KIR3DL1/3DS1 to ankylosing spondylitis in human leukocyte antigen-B27
Caucasian populations. Arthritis Res Ther. 2006;8(4):R101.
33. Matzkies FG, Targan SR, Berel D, Landers CJ, Reveille JD, McGovern DP, et al. Markers of intestinal inflammation in patients with ankylosing spondylitis: a pilot
study. Arthritis Res Ther. 2012 Nov;14(6):R261.
34. Togrol RE, Nalbant S, Solmazgül E, Ozyurt M, Kaplan M, Kiralp MZ, et al. The significance of coeliac disease antibodies in patients with ankylosing spondylitis:
a case-controlled study. J Int Med Res. 2009 Jan-Feb;37(1):220–6.
35. Skare TL, Bortoluzzo AB, Gonçalves CR, Braga da Silva JA, Ximenes AC, Bértolo MB, et al. Ethnic influence in clinical and functional measures of Brazilian patients with spondyloarthritis. J Rheumatol. 2012 Jan;39(1):141–7.
36. Wei JC, Hsu YW, Hung KS, Wong RH, Huang CH, Liu YT, et al. Association study of polymorphisms rs4552569 and rs17095830 and the risk of ankylosing
spondylitis in a Taiwanese population. PLoS One. 2013;8(1):e52801
37. Duarte AP, Marques CD, Bortoluzzo AB, Gonçalves CR, da Silva JA, Ximenes AC, et al. [Epidemiologic profile of juvenile-onset compared to adult-onset spondyloarthritis
in a large Brazilian cohort]. Rev Bras Reumatol. 2014 Nov-Dec;54(6):424–30. Portuguese
38. D’Incà R, Podswiadek M, Ferronato A, Punzi L, Salvagnini M, Sturniolo GC. Articular manifestations in inflammatory bowel disease patients: a prospective study. Dig Liver Dis. 2009 Aug;41(8):565–9.
39. Tsui FW, Tsui HW, Las Heras F, Pritzker KP, Inman RD. Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing
spondylitis. Ann Rheum Dis. 2014 Oct;73(10):1873–9.
40. Riente L, Chimenti D, Pratesi F, Delle Sedie A, Tommasi S, Tommasi C, et al. Antibodies to tissue transglutaminase and Saccharomyces cerevisiae in ankylosing spondylitis
and psoriatic arthritis. J Rheumatol. 2004 May;31(5):920–4.
41. Hoffman IE, Demetter P, Peeters M, De Vos M, Mielants H, Veys EM, et al. Anti-saccharomyces cerevisiae IgA antibodies are raised in ankylosing spondylitis
and undifferentiated spondyloarthropathy. Ann Rheum Dis. 2003 May;62(5):455–9.
42. Torok HP, Glas J, Gruber R, Brumberger V, Strasser C, Kellner H, et al. Inflammatory bowel disease-specific autoantibodies in HLA-B27-associated spondyloarthropathies:
increased prevalence of ASCA and pANCA. Digestion. 2004;70(1):49–54.
43. Klingberg E, Carlsten H, Hilme E, Hedberg M, Forsblad-d’Elia H. Calprotectin in ankylosing spondylitis—frequently elevated in feces, but normal in
serum. Scand J Gastroenterol. 2012 Apr;47(4):435–44.
44. Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Tanjong Ghogomu E, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015 Apr 18;4:ECD005468.
45. Stoll ML, Kumar R, Morrow CD, Lefkowitz EJ, Cui X, Genin A, et al. Altered microbiota associated with abnormal humoral immune responses to commensal
organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014 Nov;16(6):486.
46. Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic
review and meta-analysis. J Crohns Colitis. 2014 Dec;8(12):1569–81.
47. Kohlo KL, Korpela K, Jaakkola T, Pichai MV, Zoetendal EG, Salonen A, et al. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol. 2015 Jun;110(6):921–30.
48. Karimi O, Peña AS. Indications and challenges of probiotics, prebiotics, and symbiotics in the management
of arthralgias and spondyloarthropathies in inflammatory bowel disease. J Clin Gastroenterol. 2008 Sep;42 Suppl 3 Pt 1:S136–41.
49. Lee J, Rachmilewitz D, Raz E. Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci. 2006 Aug;1072:351–5.
Jaclyn Rivington, 1 Saba University School of Medicine, Caribbean Netherlands.
Michael Gillett, 1 Saba University School of Medicine, Caribbean Netherlands.
About the Author: Jaclyn Rivington is a fourth-year medical student at Saba University School of Medicine,
graduating in 2017. She completed her undergraduate studies at Guelph University in
Canada.
Correspondence Jaclyn Rivington. Address: Saba University School of Medicine, The Bottom, Caribbean
Netherlands. Email: jaclynrivington@gmail.com
Cite as: Rivington J, Gillett M. Establishing a causal link between ankylosing spondylitis and inflammatory bowel disease: a review of the literature. Int J Med Students. 2016 May-Aug;4(2):55-63.
Copyright © 2016 Jaclyn Rivington, Michael Gillett
International Journal of Medical Students, VOLUME 4, NUMBER 2, August 2016

