Original Article
De-Epithelialization Protocol with Tapered Sodium Dodecyl Sulfate Concentrations Enhances
Short-Term Chondrocyte Survival in Porcine Chimeric Tracheal Allografts
Kevin Xiang Zhou1, Fabio Gava Aoki2, Alba Marin3, Golnaz Karoubi4, Siba Haykal5, Thomas K. Waddell6
doi: http://dx.doi.org/10.5195/ijms.2023.1437
Volume 11, Number 1: 13-21
Received 12 03 2022;
Rev-request 27 04 2022;
Rev-request 28 11 2022;
Rev-recd 03 06 2022;
Rev-recd 29 12 2022;
Accepted 01 01 2023
ABSTRACT
Background:
Tracheal transplantation is indicated in patients with extensive defects that are
unable to be repaired via primary reconstruction. However, transplantation is currently
considered a high-risk treatment option partly due to high morbidity and mortality
associated with graft rejection. Recently, decellularization (decell) has been explored
as a technique for creating bioengineered tracheal grafts. However, this method increases
risk of post-operative stenosis due to the death of chondrocytes, which are critical
to maintain the biochemical and mechanical integrity of tracheal cartilage. In this
project, we propose a novel decell protocol that adequately removes epithelial, mucosal,
and submucosal cells while maintaining a greater proportion of viable chondrocytes.
Methods:
The trachea of adult male outbred Yorkshire pigs were extracted, decontaminated, and
decellularized according to the original and new protocols before incubation at 37
°C in Dulbecco’s Modified Eagle Medium (DMEM) for 10 days. Chondrocyte viability was
quantified immediately following post-decellularization and on days 1, 4, 7, and 10.
Histology was performed pre-decell, post-decell, and post-incubation.
Results:
The new protocol showed a significant (p < 0.05) increase in chondrocyte viability
up to four days after decell when compared to the original protocol. The new protocol
also preserves extracellular matrix (ECM) composition to a similar degree as the original
protocol. When scaffolds created using the new protocol were re-epithelialized, cell
growth curves were near identical to published data from the original protocol.
Conclusion:
Despite limited improvements in long-term chondrocyte viability, the new protocol
may be used to engineer chimeric tracheal allografts without the need for cartilage
regeneration up to four days post-decellularization.
Keywords:
Tissue Engineering;
Decellularization;
Allograft;
Trachea;
Bioreactor;
Regenerative Medicine;
Chondrocyte;
Stem Cell;
Graft;
Transplantation;
Transplant;
Surgery;
Bioengineering;
Stenosis;
Cartilage;
Viability;
Cell Viability;
Medicine (Source: MeSH-NLM).
Introduction
Tracheal transplantation is a surgical procedure that aims to restore the airway in
patients with extensive defects that are unable to be repaired via primary reconstruction.
Transplantation is indicated in cases where injury exceeds 50% of the organ in adults
and 30% in children.1 However, tracheal replacement therapy is currently considered a high-risk procedure,
and is mostly offered as a treatment option in compassionate use cases. A major reason
behind the relatively high rate of complications is the plethora of immunological
compatibility issues created by orthotopically transplanting a donor organ.2 A possible solution to this problem may be found in tissue engineering-based approaches
for whole-trachea regeneration. Recently, significant progress has been made in engineering
bioartificial organs de novo from pluripotent stem cells and acellular extracellular
matrix (ECM) scaffolds.3–6 Somatic cells have been differentiated into functional lung epithelial cells after
transformation into induced pluripotency.7 Also, stem cell-seeded tracheal grafts from cadaveric donors have been transplanted
into patients with end-stage airway diseases.4 Despite these milestones, recellularized tracheal allografts still demonstrate increased
risk of stenosis, resulting in post-operative complications.3,4,8
Decellularization (decell) of donor trachea is a relatively well-studied technique
for creating natural scaffolds for whole-trachea regeneration.9–15 One such decell approach involves the use of detergents to remove donor cells from
a cadaveric trachea, leaving behind the ECM scaffold.11,16,17 Recipient-derived induced pluripotent stem cells (iPSCs) may then be seeded onto
such scaffold, thereby reconstituting the respiratory epithelium.4 The benefits of this approach are twofold. Firstly, risk of graft rejection is reduced
because the immunogenic donor tracheal epithelium and submucosa are removed and replaced
with autologous cells.8,10,18–20 Secondly, the use of a native biological scaffold rather than synthetic materials
preserves the important tissue architecture and ultrastructure, which allows for greater
mimicking of the cellular niche later during scaffold seeding.17 However, the full thickness decell protocols currently in use are harmful to chondrocytes,
leading to deficiencies in the biochemical and mechanical integrity of hyaline cartilage.14,17,21 This may increase the risk of post-operative stenosis and other complications upon
implantation.22 To address this issue, the Waddell lab uses a de-epithelialization (de-ep) technique
pioneered by Aoki et al. in 2019 to remove only the immunogenic epithelium while maintaining
chondrocyte viability.17,23 This de-ep technique can be followed by re-epithelialization (re-ep) using autologous
cells to produce chimeric tracheal allografts.
Despite these advances, the original de-ep protocol is suboptimal due to relatively
low chondrocyte survival rates (68.6 ± 7.3%).17 A new de-ep protocol has recently been developed by the Waddell lab based on the
postulated chemical and osmotic effects of various decellularization agents on chondrocytes.
This protocol is believed to provide milder de-ep conditions that may increase chondrocyte
survival while providing similar removal of epithelial cells. When designing this
new protocol, the following hypotheses were made: 1) removal of the standard 40 minute
ddH2O wash cycle will decrease osmotic stress on cells perforated by sodium dodecyl sulfate
(SDS) detergent, the most common decellularization agent used in previous protocols,
and 2) using decreasing concentrations of SDS rather than a static concentration will
remove greater amounts of residual SDS in submucosal tissue, thus protecting cartilage.
An initial high concentration (1%) is required for decellularizing epithelium and
mucosa, after which lower concentrations of SDS (0.1%, 0.01%) are more appropriate
for minimizing damage to cartilage. This study intends to serve as a proof-of-concept
to demonstrate that a modified de-ep protocol can allow the removal of immunogenic
tissue (epithelium, mucosa, submucosa, and perichondrium) while preserving a greater
portion of the chondrocyte population. The objectives of this study are to: 1) evaluate
chondrocyte viability in porcine trachea after the use of the new de-ep protocol,
2) evaluate the preservation of ECM biochemical composition after the new protocol,
and 3) evaluate the degree of epithelial cell attachment and viability during re-ep
after the new protocol. We hypothesize that the new protocol will produce de-epithelialized
scaffolds with improved chondrocyte viability while demonstrating similar biochemical
composition and re-epithelialization performance as compared to the current protocol.
Methods
Tracheal extraction
Adult male outbred Yorkshire pigs (30-40 kg) (n = 18) sourced from the University
Health Network (UHN) Animal Resources Centre were used as donor animals due to the
physiological similarity of their cardiopulmonary system to that of humans. After
anesthesia by isoflurane administration, a median incision of the neck was made to
expose the larynx and upper trachea. Next, a median sternotomy was performed to open
the chest wall and provide access to the lower trachea. Using Mayo scissors, the trachea
was bisected just below the cricothyroid membrane and lifted away from the esophagus.
Surrounding connective tissue was dissected away using curved Mayo scissors. To detach
the trachea, the left and right main bronchus were bisected just below the carina.
The extracted trachea was immediately placed in decontamination solution at 0 °C until
transported out of the operating room. The decontamination solution contained Hank’s
balanced salt solution (HBSS, ThermoFisher, USA) supplemented with 2% (w/v) bovine
serum albumin (BSA, ThermoFisher, USA), fluconazole (4 μg/mL, Gibco, USA), colistimethate
(5 μg/mL, Gibco, USA), imipenem/cilastatin (25 μg/mL, Gibco, USA), ceftazidime (154
μg/mL, Gibco, USA), penicillin (200 U/mL, Gibco, USA), streptomycin (200 μg/mL, Gibco,
USA), amphotericin B (2.5 μg/mL, Gibco, USA) and gentamicin (50 μg/mL, Gibco, USA).
The tracheas were subsequently incubated at room temperature on a rocking platform
(30rpm) for 2 hours. After this incubation, the decontamination solution was replaced
with fresh solution, and luminal mucus was scraped off using a micro-tapered stainless-steel
spatula. The tracheas were incubated at 4 °C overnight until de-ep was performed the
next morning.
Animals selected for tracheal extraction surgery were screened against the following
exclusion criteria:
- Respiratory pathologies,
- Participation in concomitant respiratory studies (ex: bleomycin lung injury model),
and
- More than 30 minutes since cardiac death (donor warm ischemia time).
All animals received humane care in compliance with the “Principles of Laboratory
Animal Care” formulated by the National Society for Medical Research and the “Guide
for the Care of Laboratory Animals” published by the National Institutes of Health.
The study was approved by the Animal Care Committee of the Toronto General Research
Institute.
De-epithelialization and incubation
The following solutions were prepared under sterile conditions and adjusted to a pH
of 7.4: 1%, 0.1%, and 0.01% SDS; 1% triton X-100; Dulbecco’s phosphate buffered saline
(DPBS). A perfusion system was constructed using polyvinyl chloride (PVC) tubing and
4-way Luer connection stopcocks as illustrated in Figure 1. A rotating perfusion bioreactor was used, modified from Haykal et al. Using three
2/0 silk sutures, the trachea was anastomosed to the bioreactor with its proximal
end facing the inlet of the chamber (Figure 2).4 De-ep was performed according to the original, new, and control protocols outlined
in Tables 1–2.17
Figure 1.
A: The Perfusion Circuitry Designed for the Original de-ep Protocol.17 Order of Perfusion is Numbered from 1–4 and Corresponds to the Solutions in Table 1. B: The Perfusion Circuitry Designed for the new de-ep Protocol. Order of Perfusion
is Numbered from 1–5 and Corresponds to the Solutions in Table 2.

Figure 2.
Timepoints for live/dead Staining and Histology used in this Study. Bottom left: Appearance
of the Bioreactor with lid Removed. Trachea is Visible, Surrounded by Dulbecco’s Modified
Eagle Medium (DMEM).

Table 1.
Original de-epithelialization Protocol.17
| Step |
Reagents* |
Time |
Vol. (mL) |
pH |
Temp. (°C) |
| 1† |
1% SDS |
3 hr |
75 |
7.4 |
37 |
| 2† |
ddH2O
|
30 min |
140 |
7.4 |
37 |
| 4‡ |
1% Triton |
30 min |
140 |
7.4 |
37 |
| 5‡ |
DPBS (-/-) |
30 min |
140 |
7.4 |
37 |
Legend:
*Reagents inside trachea (Lumen). Outside the trachea, DMEM with 10% FBS + 1% Penicillin-Streptomycin
solution remains circulating.
†De-epithelialization process – pulsatile perfusion
‡Washing steps – continuous perfusion
DMEM = Dulbecco’s Modified Eagle Medium
SDS = Sodium Dodecyl Sulfate
DPBS = Dulbecco’s Phosphate-Buffered Saline
Table 2.
New De-Epithelialization Protocol.
| Step |
Reagents* |
Time |
Vol. (mL) |
pH |
Temp. (°C) |
| 1† |
1% SDS |
1 hr |
75 |
7.4 |
37 |
| 2† |
0.1% SDS |
1 hr |
75 |
7.4 |
37 |
| 3† |
0.01% SDS |
1 hr |
75 |
7.4 |
37 |
| 4‡ |
1% Triton |
30 min |
140 |
7.4 |
37 |
| 5‡ |
DPBS (-/-) |
30 min |
140 |
7.4 |
37 |
Legend:
*Reagents inside trachea (Lumen). Outside the trachea, DMEM with 10% FBS + 1% Penicillin-Streptomycin
solution remains circulating
†De-epithelialization process – pulsatile perfusion
‡Washing steps – continuous perfusion
DMEM = Dulbecco’s Modified Eagle Medium
SDS = Sodium Dodecyl Sulfate
DPBS = Dulbecco’s Phosphate-Buffered Saline
Following de-ep, the proximal and distal ends of the trachea were trimmed such that
only the portions exposed to the decellularization media were used for the subsequent
10-day incubation. The tracheal segments were then placed in decontamination solution
for 48 hours at 4 °C on a rocking platform (30 rpm). Finally, the tracheae were incubated
at 47 °C with 5% CO2 (ThermoFisher, USA) in a 250 mL Erlenmeyer flask fitted with
a 20-micron filter allowing for gas exchange. The media used was Dulbecco’s Modified
Eagle Medium (DMEM, Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (FBS,
Gibco, USA), fluconazole (4 µg/mL), colistimethate (5 μg/mL), imipenem/cilastatin
(25 μg/mL, Gibco, USA), ceftazidime (154 μg/mL, Gibco, USA), penicillin (200 μg/mL,
Gibco, USA), streptomycin (200 μg/mL, Gibco, USA), amphotericin B (2.5 μg/mL, Gibco,
USA) and gentamicin (50 μg/mL, Gibco, USA). Media was changed every 48 hours.
To accurately compare the two de-ep protocols being tested, two negative control groups
were employed. The first control was a decontaminated native trachea that immediately
underwent static incubation for ten days without any de-ep procedure, henceforth referred
to as “control-native.” The second control was exposed to the same conditions as the
trachea that underwent the new protocol, except with DPBS replacing all steps that
required SDS, henceforth referred to as “control-DPBS” (Table 3).
Table 3.
Control – New De-Epithelialization Protocol without SDS. Identical Conditions as new
Protocol, Except Perfused with DPBS Instead of SDS for Preparation of Control-DPBS
Trachea.
| Step |
Reagents* |
Time |
Vol. (mL) |
pH |
Temp. (°C) |
| 1† |
1% DPBS |
1 hr |
75 |
7.4 |
37 |
| 2† |
1% DPBS |
1 hr |
75 |
7.4 |
37 |
| 3† |
1% DPBS |
1 hr |
75 |
7.4 |
37 |
| 4‡ |
1% Triton |
30 min |
140 |
7.4 |
37 |
| 5‡ |
DPBS (-/-) |
30 min |
140 |
7.4 |
37 |
Legend:
*Reagents inside trachea (Lumen). Outside the trachea, DMEM with 10% FBS + 1% Penicillin-Streptomycin
solution remains circulating.
†De-epithelialization process − pulsatile perfusion.
‡Washing steps − continuous perfusion. DMEM = Dulbecco's Modified Eagle Medium. SDS
= Sodium Dodecyl Sulfate. DPBS = Dulbecco's Phosphate-Buffered Saline
Two control groups are necessary to rule out any potential negative effects on chondrocyte
viability arising from tracheal harvesting and installation into the bioreactors.
If both controls demonstrate similar levels of near-100% viability, the study can
conclude that the primary determinant of chondrocyte viability is the protocol itself,
in other words, the series of SDS decellularization steps. Three biological replicates
– each consisting of a single trachea harvested from a random Yorkshire pig – were
performed for the original protocol, the new protocol, and the two control groups.17 Day 0 was defined as the start of the bioreactor incubation period, hence day −2
was when the decellularization protocol was performed. Figure 3 illustrates the study protocol as a flowchart diagram.
Figure 3.
The Experimental Protocol Followed in the Current Study, Illustrated as a Flowchart
Diagram.

Histological analysis
Histological samples were taken from the trachea before de-ep, after de-ep, and after
incubation (Figure 4). Specimens were fixed with 4% paraformaldehyde for 24 hours and processed with an
automated vacuum tissue processor (Leica, USA). Tissue was sectioned into 5 μm slices
and stained with hematoxylin and eosin (H&E), Masson’s trichrome (Sigma-Aldrich, USA),
Verhoeff’s elastin (Sigma-Aldrich, USA), and Alcian blue (NovaUltra™, IHC World, USA).
Figure 4.
Chondrocyte Viability Following De-epithelialization and 10-day Incubation in Static
Media. Statistically Significant Differences as Determined by a Two-Way Analysis of
Variance (ANOVA) with Tukey’s Post Hoc Multiple Comparisons Test are Indicated. P-Values
Given as: <0.0332 = *, <0.0021 = **, <0.0002 = ***, <0.0001 = ****

Quantification of chondrocyte viability
Chondrocyte viability was quantified immediately after de-ep and on days 1, 4, 7,
and 10 (Figure 3). Two to three rings were obtained from each trachea for a membrane integrity-based
viability assay. The mucosa and submucosa were dissected away from the cartilage using
fine forceps. The cartilage ring was opened and manually cut in cross section into
thin (<1 mm) slices. An ethidium homodimer assay (LIVE/DEAD™ Viability/Cytotoxicity
Kit, Invitrogen, USA) was performed as per manufacturer directions. The slices were
imaged under confocal microscopy at 20x magnification (A1R, Nikon, Japan). Images
were then examined manually by a blinded experimenter. Portions of the image containing
viable chondrocytes were circumscribed and the area was calculated. The percentage
viability of an image was calculated through the following formula:

Three technical replicates were performed per trachea.
Re-epithelialization
The de-ep bioreactor circuitry from Haykal et al. was modified to include media reservoirs
for oxygenation, in addition to syringe ports for media changes and sample collection.4 A 1 mL suspension of BEAS-2B human bronchial epithelial cells (~1×106 cells/cm2) was injected into the lumen. Cells were allowed to adhere for 2 hours under bidirectional
flow at a rate of 1.5 mL/min. After the initial 2 hours, we started unidirectional
perfusion of the lumen at the same rate for seven days. During re-ep, media in the
luminal circuit (30 mL) was changed every 24 hours and media in the outer circuit
(250 mL) was changed every 48 hours.
Cell proliferation activity assay
Cell proliferation during re-ep was measured using a resazurin-based cell viability
assay as per manufacturer instructions (PrestoBlue®, Invitrogen, USA). Briefly, a
20 mL solution of 1:20 (v/v) PrestoBlue/DMEM + 10% FBS was prepared. Three 0.5 mL
volumes were separated for use as a negative control. The remaining 18.5 mL of reagent
was injected into the luminal perfusion circuit of the bioreactor and allowed to circulate
for 1 hr. Afterwards, the PrestoBlue solution was aspirated out of the luminal circuit
and aliquoted into three 0.5 mL replicates in a 24-well plate for fluorescence analysis
at 560 nm (Cytation™ 5, BioTek Instruments).
Statistical analysis
Commercial statistical software (GraphPad Software Inc., USA) was used for statistical
analysis. A 2-way analysis of variance (ANOVA) was used to determine statistically
significant differences (p ≤ .05) between the three protocols (original, new, control-DPBS),
with Tukey’s post hoc multiple comparisons test. Values in figures are presented as
means with standard deviations (SD).17
Results
Quantification of chondrocyte viability
There exists an overall negative correlation between days since de-ep and percentage
chondrocyte viability (Figure 4). Both the original and new protocols significantly reduce viability compared to
the control protocol (no SDS) and unprocessed native trachea.17 However, the new protocol provides significantly (p = 0.0069) improved viability
compared to the original protocol in the first four days, after which there is no
detectable difference.17 The most marked improvement in chondrocyte viability occurs on day 4 (61.3±10.8%
vs 40.7±5.7%), yet the benefit of the new protocol towards chondrocytes is seen as
early as immediately after de-ep on day −2 (78.1±4.7% vs 61.5±10.7%). In other words,
long-term chondrocyte survival remains unchanged. Qualitative inspection of live/dead
staining reveals the most chondrocyte death at the luminal surface of each cartilage
ring (Figure 5). There appears to be a smaller “wavefront” of chondrocyte death in the new protocol
compared to the original protocol. The average chondrocyte viability of two replicates
(n=2) after a 7-day re-ep was 63%.
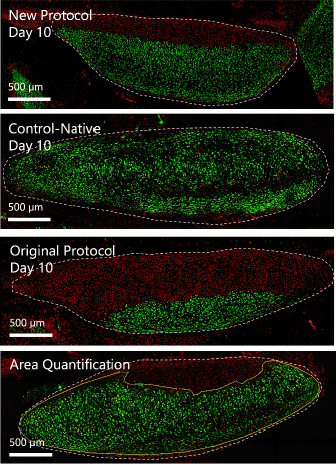
Figure 5.
Chondrocyte Viability in new Protocol, Original Protocol, and Control-Native Trachea
on day 10 of Static Incubation.17 Confocal Microscopy Images Depicting Calcein-AM for live (Green) and Ethidium Homodimer-1
for dead (Red) Cells in Cross-Sections of Cartilage Rings (Marked as the area within
the White Dotted Line). Bottom Right Image Shows the Calculation for Percentage Viability
as the area within the Solid Yellow Line (Live Cells) Divided by area within the White
Dotted Line (Total Cross-Sectional Area).
Histological analysis
In the native trachea control, H&E staining showed the expected pseudostratified columnar
epithelium with cilia and goblet cells (Figure 6). In both the original and new de-ep protocols, H&E showed a denuded epithelium,
with no residual cellular material.17 No nuclei or cytosolic elements were found in the epithelium. However, both protocols
resulted in some nuclei remaining in the deep submucosal regions. Residual acinar
gland cells were also visible in both protocols. The hyaline cartilage appears morphologically
unchanged.
Figure 6.
A) Masson’s trichrome stain B) Verhoeff’s elastin stain C) Alcian blue stain D) Hematoxylin and eosin stain of control-native; trachea processed with the original/current
de-ep protocol; and trachea processed with the new de-ep protocol.17 10x and 60x magnifications are shown in the top and bottom rows respectively. The
lumen (L), epithelium (E), submucosa (SM), acinar glands (AG) and hyaline cartilage
(HC) are labelled.
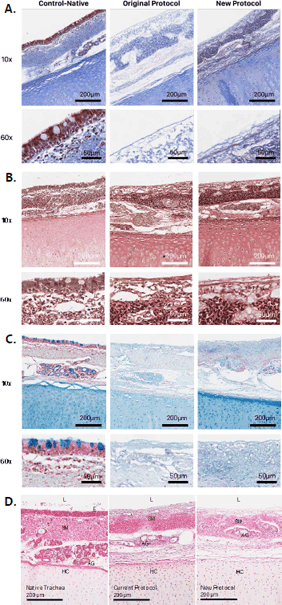
Masson’s trichrome stain showed good collagen preservation throughout the ECM in both
the original and new protocols (Figure 6).17 Keratin fibers in the deep submucosa appear better preserved in the new protocol.
Verhoeff’s elastin stain showed good preservation of elastin fibers in the mucosa
and submucosa of both the original and new de-ep protocols (Figure 6).17 Alcian blue stain showed good preservation of acidic polysaccharides such as glycosaminoglycans
in cartilage, in both the original and new protocols (Figure 6).17
Cell proliferation activity assay
When the new protocol’s re-ep cell proliferation curve is compared with that of the
original protocol from Aoki et al., there is similarity in the rate at which fluorescence
increases (Figure 7).17 The difference between the two growth curves is nonsignificant (p=0.15, 0.59, 0.86,
0.59, 0.89, 0.20, 0.84) as indicated by a multiple t test (false discovery rate approach).
Although not a focus of this study, chondrocyte viability after the 7-day re-ep with
BEAS-2B was evaluated with two tracheae. The average chondrocyte viability was 63%.
Figure 7.
Growth Curves of BEAS-2B on the New and Old Protocol’s Scaffolds over Seven-Day Re-Epithelialization
Period.

Legend: p = 0.15, 0.59, 0.86, 0.59, 0.89, 0.20, 0.84.
Discussion
It has been demonstrated in previous literature that SDS reduces cell viability by
acting as an anionic detergent, perforating the cell membrane and causing osmotic
lysis.11,12,22 The original protocol contains a 3 hour 1% SDS wash that can leave residual detergent
trapped in tissue, thus causing ongoing damage after the protocol is terminated.17 Furthermore, the original protocol includes a 30 minute ddH2O wash that can cause further chondrocyte death via osmotic imbalance leading to cytolysis.17 The new protocol made two changes to the original protocol: 1) the 3 hour SDS cycle
has been replaced with three 1 hour cycles at decreasing SDS concentrations (1%, 0.1%,
0.01%), and 2) The 30 minute ddH2O wash has been removed.17 It is believed that the first change limits deep penetration of residual SDS into
tissue, while the second change reduces cytolysis of chondrocytes. In other words,
this new protocol was designed to provide milder de-ep conditions that increase chondrocyte
survival while providing similar removal of epithelial cells. Both negative controls
(control-native and control-DPBS) showed close to 90% viability. Therefore, it seems
that SDS retention in the ECM is a major contributor to chondrocyte death after de-ep,
overshadowing the cytolytic effect of the ddH2O wash and other potential minor contributors. Attempts at quantifying the amount
of residual SDS in de-epithelialized tissues using a methylene blue assay were unsuccessful.
Future studies should investigate the relationship between residual SDS levels and
chondrocyte viability. The short-term nature of the improvement in chondrocyte viability
observed in this study was likely due to an initial reduction in residual SDS concentration
in submucosal tissues, followed by eventual permeation of the SDS through submucosa
and into cartilage due to passive diffusion. Confocal images of the cell viability
assay show a clear delineation between calcein-AM (live cells) and ethidium homodimer-1
(dead cells), suggesting a progressive “wavefront” of cell death that is consistent
with diffusion of residual SDS. Confirmation of this theory is required, although
preventing the diffusion of SDS through submucosal tissue would be difficult or impractical
to accomplish in any de-ep protocol.
Examination of H&E slides shows that both protocols were extremely efficient at denuding
the epithelium. However, neither protocol appears to sufficiently decellularize acinar
glands. Furthermore, the new protocol seems to be less efficient at decellularizing
deep submucosal layers. This result was expected since the new protocol uses decreasing
concentrations of SDS and is less aggressive overall compared to the original protocol,
among others.17,24,25 Therefore, with the current detergent-based methods of de-ep, the goal of selectively
preserving chondrocyte viability seems to depend on the careful titration of SDS concentrations,
walking a fine balance between over- and under-decellularization. The current study
shows that the new protocol sacrifices decellularization performance in return for
better chondrocyte survival.
Previous studies have shown that decellularization cycles can reduce several ECM components
that are critical to structural integrity, including elastin, collagen, and glycosaminoglycans.17,25,26 Qualitative histological analysis demonstrated that the new protocol is not any more
damaging to ECM components than the original protocol.17 Elastin, collagen, and glycosaminoglycans were found to be preserved after de-ep
to a similar degree as with the original protocol.17 Tracheal compliance and viscoelasticity were not tested because previous studies
by Aoki et al. have confirmed no difference in these mechanical properties after the
more aggressive original de-ep protocol.17
The cellular proliferation assay suggests that the new protocol has no negative effects
on metabolism and growth of the BEAS-2B cells used for re-ep. This suggests that ECM
scaffolds created using the new de-ep protocol can support epithelial cell attachment
and viability during re-ep, allowing for the creation of chimeric allografts.
This proof-of-concept study is not without limitations. To longitudinally measure
chondrocyte survival, we incubated the de-epithelialized trachea in static Dulbecco’s
Modified Eagle Medium (DMEM) to simulate implantation of the grafts. This does not
fully recapitulate the complex cell-environment interactions present in vivo. Therefore,
conclusions regarding chondrocyte viability should be validated in a bioreactor environment
that simulates nutrient perfusion, hydrodynamic stimuli, and mechanical stimuli.27,28 The current study did evaluate chondrocyte viability of de-epithelialized trachea
after a 7-day re-ep in a double-chamber bioreactor, yielding a percentage viability
of 63% over 7-days. This result is promising given that previous studies have demonstrated
that a 50% chondrocyte viability was associated with successful tracheal transplantation
in dogs, with no lethal stenosis.29 However, future studies should be conducted with a larger number of replicates.
In conclusion, we introduce a new de-ep protocol with improved short-term chondrocyte
viability. The results of this study have indicated that improvements in the protocol
can still be made. However, the data presented sheds light on the potential mechanism
of chondrocyte death during and after de-ep.
Acknowledgments
None.
Conflict of Interest Statement & Funding
The Authors have no financial relationships or conflicts of interest to disclose.
This research was undertaken thanks in part to funding from the Canada First Research
Excellence Fund and the IMS SURP program.
Author Contributions
Conceptualization: KXZ, FGA, GK, SH, TKW. Data Curation: KXZ. Formal Analysis: KXZ.
Funding Acquisition: GK, TKW. Investigation: KXZ, FGA, AM. Methodology: KXZ, FGA.
Project Administration: KXZ, FGA, GK, SH, TKW. Resources: KXZ. Software: KXZ. Supervision:
FGA, AM, GK, SH, TKW. Validation: KXZ, FGA. Visualization: KXZ. Writing - Original
Draft: KXZ. Writing - Review Editing: KXZ.
References
1. Etienne H, Fabre D, Gomez Caro A, Kolb F, Mussot S, Mercier O, et al. Tracheal replacement. Eur Respir J. 2018;51(2):1702211.
2. Lama VN, Belperio JA, Christie JD, El-Chemaly S, Fishbein MC, Gelman AE, et al. Models of Lung Transplant Research: a consensus statement from the National Heart,
Lung, and Blood Institute workshop. JCI Insight. 2017;2(9):e93121.
3. Elliott MJ, Butler CR, Varanou-Jenkins A, Partington L, Carvalho C, Samuel E, et al. Tracheal Replacement Therapy with a Stem Cell-Seeded Graft: Lessons from Compassionate
Use Application of a GMP-Compliant Tissue-Engineered Medicine. Stem Cells Transl Med. 2017;6(6):1458–64.
4. Haykal S, Salna M, Waddell TK, Hofer SO. Advances in Tracheal Reconstruction. Plast Reconstr Surg Glob Open. 2014;2(7):e178.
5. Wang Y, Bao J, Wu Q, Zhou Y, Li Y, Wu X, et al. Method for perfusion decellularization of porcine whole liver and kidney for use as
a scaffold for clinical-scale bioengineering engrafts. Xenotransplantation. 2015;22(1):48–61.
6. Varma R, Soleas JP, Waddell TK, Karoubi G, McGuigan AP. Current strategies and opportunities to manufacture cells for modeling human lungs. Adv Drug Deliv Rev. 2020;161–162:90–109.
7. Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, et al. Generation of Multipotent Lung and Airway Progenitors from Mouse ESCs and Patient-Specific
Cystic Fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–97.
8. Liu Y, Nakamura T, Sekine T, Matsumoto K, Ueda H, Yoshitani M, et al. New Type of Tracheal Bioartificial Organ Treated with Detergent: Maintaining Cartilage
Viability Is Necessary for Successful Immunosuppressant Free Allotransplantation. ASAIO J. 2002;48(1):21–5.
9. Conconi MT, Coppi PD, Liddo RD, Vigolo S, Zanon GF, Parnigotto PP, et al. Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the
adhesion of chondrocytes and tracheal epithelial cells. Transpl Int. 2005;18(6):727–34.
10. Jungebluth P, Go T, Asnaghi A, Bellini S, Martorell J, Calore C, et al. Structural and morphologic evaluation of a novel detergent–enzymatic tissue-engineered
tracheal tubular matrix. Journal Thorac Cardiovasc Surg. 2009;138(3):586–93.
11. Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem. 2012;113(7):2217–22.
12. Gilbert T, Sellaro T, Badylak S. Decellularization of tissues and organs. Biomaterials. 2006;S0142961206001682.
13. Weymann A, Patil NP, Sabashnikov A, Korkmaz S, Li S, Soos P, et al. Perfusion-Decellularization of Porcine Lung and Trachea for Respiratory Bioengineering:
Bioartificial Lungs and Tracheae. Artif Organs. 2015;39(12):1024–32.
14. Hung SH, Su CH, Lin SE, Tseng H. Preliminary experiences in trachea scaffold tissue engineering with segmental organ
decellularization: Segmental Trachea Decellularization Tissue Engineering. Laryngoscope. 2016;126(11):2520–7.
15. Hung SH, Su CH, Lee FP, Tseng H. Larynx Decellularization: Combining Freeze-Drying and Sonication as an Effective Method. J Voice. 2013;27(3):289–94.
16. Cebotari S, Tudorache I, Jaekel T, Hilfiker A, Dorfman S, Ternes W, et al. Detergent Decellularization of Heart Valves for Tissue Engineering: Toxicological
Effects of Residual Detergents on Human Endothelial Cells. Artif Organs. 2010;34(3):206–10.
17. Aoki FG, Varma R, Marin-Araujo AE, Lee H, Soleas JP, Li AH, et al. De-epithelialization of porcine tracheal allografts as an approach for tracheal tissue
engineering. Sci Rep. 2019;9(1):12034.
18. Zang M, Zhang Q, Chang EI, Mathur AB, Yu P. Decellularized Tracheal Matrix Scaffold for Tracheal Tissue Engineering: In Vivo Host
Response. Plast Reconstr Surg. 2013;132(4):549e–59e.
19. Liu Y, Nakamura T, Yamamoto Y, Matsumoto K, Sekine T, Ueda H, et al. Immunosuppressant-free allotransplantation of the trachea. J Thorac Cardiovasc Surg. 2000;120(1):108–14.
20. Liu Y, Nakamura T, Yamamoto Y, Matsumoto K, Sekine T, Ueda H, et al. A New Tracheal Bioartificial Organ: Evaluation of a Tracheal Allograft with Minimal
Antigenicity after Treatment by Detergent: ASAIO J. 2000;46(5):536–9.
21. Remlinger NT, Czajka CA, Juhas ME, Vorp DA, Stolz DB, Badylak SF, et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010;31(13):3520–6.
22. Gilpin A, Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques
to Applications. BioMed Res Int. 2017;2017:1–13.
23. Marin-Araujo AE, Haykal S, Karoubi G. Bioreactor-Based De-epithelialization of Long-Segment Tracheal Grafts. Methods Mol Biol. 2022;2436:167-182
24. Haykal S, Zhou Y, Marcus P, Salna M, Machuca T, Hofer SOP, et al. The effect of decellularization of tracheal allografts on leukocyte infiltration and
of recellularization on regulatory T cell recruitment. Biomaterials. 2013;34(23):5821–32.
25. Haykal S, Soleas JP, Salna M, Hofer SOP, Waddell TK. Evaluation of the Structural Integrity and Extracellular Matrix Components of Tracheal
Allografts Following Cyclical Decellularization Techniques: Comparison of Three Protocols. Tissue Eng Part C: Methods. 2012;18(8):614–23.
26. Partington L, Mordan NJ, Mason C, Knowles JC, Kim HW, Lowdell MW, et al. Biochemical changes caused by decellularization may compromise mechanical integrity
of tracheal scaffolds. Acta Biomater. 2013;9(2):5251–61.
27. Asnaghi MA, Jungebluth P, Raimondi MT, Dickinson SC, Rees LEN, Go T, et al. A double-chamber rotating bioreactor for the development of tissue-engineered hollow
organs: From concept to clinical trial. Biomaterials. 2009;30(29):5260–9.
28. Lee H, Marin-Araujo AE, Aoki FG, Haykal S, Waddell TK, Amon CH, et al. Computational fluid dynamics for enhanced tracheal bioreactor design and long-segment
graft recellularization. Sci Rep. 2021;11(1):1187.
29. Lu T, Huang Y, Qiao Y, Zhang Y, Liu Y. Evaluation of changes in cartilage viability in detergent-treated tracheal grafts
for immunosuppressant-free allotransplantation in dogs. Eur J of Cardiothorac Surg. 2018;53(3):672–9.
Kevin Xiang Zhou, 1 BMSc (Hons.). Schulich School of Medicine and Dentistry, 1151 Richmond St, London,
ON N6A 5C1, Canada.
Fabio Gava Aoki, 2 PhD, MASc. Latner Thoracic Surgery Research Laboratories, Division of Thoracic Surgery,
University Health Network, 101 College St., Toronto, ON M5G 1L7, Canada.
Alba Marin, 3 MD. Latner Thoracic Surgery Research Laboratories, Division of Thoracic Surgery,
University Health Network, 101 College St., Toronto, ON M5G 1L7, Canada.
Golnaz Karoubi, 4 PhD. Latner Thoracic Surgery Research Laboratories, Division of Thoracic Surgery,
University Health Network, 101 College St., Toronto, ON M5G 1L7, Canada.
Siba Haykal, 5 MD, PhD, FRCS(C), FACS. Latner Thoracic Surgery Research Laboratories, Division of
Thoracic Surgery, University Health Network, 101 College St., Toronto, ON M5G 1L7,
Canada.
Thomas K. Waddell, 6 MD, PhD, MSc, FRCSC. Latner Thoracic Surgery Research Laboratories, Division of Thoracic
Surgery, University Health Network, 101 College St., Toronto, ON M5G 1L7, Canada.
About the Author: Kevin Xiang Zhou is currently a second-year student at the Schulich School of Medicine
& Dentistry, Western University. He completed his BMSc (Hons) at Western University
in the department of Pathology and Laboratory Medicine.
Correspondence: Kevin Xiang Zhou. Address: 1151 Richmond St, London, ON N6A 5C1, Canada. Email: kzhou54@uwo.ca
Editor: Francisco J. Bonilla-Escobar
Student Editors: Muhammad Romail
Manan, Richard Christian Suteja & Kiera Liblik
Copyeditor: Michael V. Tavolieri
Proofreader: Laeeqa Manji
Layout Editor: Ana Maria Morales
Process: Peer-reviewed
Cite as
Zhou KX, Aoki FG, Marin A, Karoubi G, Haykal S, Waddell TK. De-Epithelialization Protocol
with Tapered Sodium Dodecyl Sulfate Concentrations Enhances Short-Term Chondrocyte
Survival in Porcine Chimeric Tracheal Allografts. Int J Med Stud. 2023 Jan-Mar;11(1):13-21.
Copyright © 2023 Kevin Xiang Zhou, Fabio Gava Aoki, Alba Marin, Golnaz Karoubi, Siba
Haykal, Thomas K. Waddell
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 11, NUMBER 1, March 2023







