Changes in Body Weight of Mice Following Lead (II) Chloride or Sodium Selenite Injections.
Isabel Sá1, Tânia Nogueira1, Elisabete Cunha2
doi: http://dx.doi.org/10.5195/ijms.2015.122
Volume 3, Number 2: 83-87
Received 03 03 2015: Accepted 20 07 2015
ABSTRACT
Background:Melanoma is a malignant skin cancer and is one of the most aggressive malignancies in humans. Heavy metals, including lead, are known to cause cellular toxicity and have been studied for their potentials to induce apoptosis in tumor cells. Since selenium is considered to act protectively in cases of lead poisoning, this study focused on the effects of sodium selenite and lead chloride, both alone and combined, on melanoma cell apoptosis.
Methods:This study was carried out by doing cell culture of melanoma cells (B16-F10 cell line) and using C57BL/6 mice. Melanoma cells suspended in lead (II) chloride, sodium selenite, or lead (II) chloride + sodium selenite solutions were injected subcutaneously to mice to induce tumor growth. After 12 days, tumors were excised and measured, followed by flow cytometry and a statistical analysis using a one-way ANOVA.
Results:In the group of mice receiving a single injection of melanoma cells suspended in 10 μmol/l of lead (II) chloride, the growth of tumor was significantly slower than in the control group. In mice treated with lead (II) chloride 50 μmol/l and 100 μmol/l, no tumor was visible at the end of the experiment. With a single injection of lead (II) chloride and sodium selenite at concentrations ≥ 10 μmol/l, the weight and size of the tumor were substantially smaller than in the control group.
Conclusion:The effect of lead (II) chloride on melanoma induction is dependent on the concentration of lead (II) chloride. Future applications may include the use of lead (II) chloride to increase apoptosis and necrosis in tumor cells and thus suppress tumor cells proliferation.
Keywords: Selenium; Lead; Melanoma; Apoptosis; Necrosis.
Modern life and industrialization bring comfort and convenience, but also entail the increase of exposure to harmful substances, including heavy metals. Different metals induce different conditions, depending on the concentration and the type of exposure.1 Heavy metals are considered to be toxic due to their ability to induce a variety of deleterious effects. The toxicity caused by heavy metals, such as arsenic (As), mercury (Hg) and lead (Pb), has already been recognized by health authorities as occupational health hazards.
Heavy metals are chemically reactive and bioaccumulative, which means that the human body is not able to effectively eliminate them, differing them from other potential toxic agents. The consequences of exposures to lead, cadmium (Cd), and mercury and the consequent pathological changes in the liver, kidney and bone are well documented.2–10 Moreover, thorium (Th), cadmium, lead, chromium (Cr), nickel (Ni), and beryllium (Be) are heavy metals with confirmed carcinogenic effects in animals and humans.11-15 Thus, there is an increasing evidence that the exposure to heavy metals could be associated with the occurrence of lung, liver, bladder, kidney, colon and skin cancer.15
One of the most widely used heavy metals is lead. This metal can enter the body through inhalation, ingestion and dermal exposure. The induction of inflammatory processes is one of the various effects caused by lead. The toxicity of lead results from its interaction with the functional groups of enzymes.16
Unfortunately, human exposure to lead is an inevitable consequence of human life, since exposures occur mainly from two sources: occupational and environmental. Lead is a toxic heavy metal which is likely to lose electrons easily, forming positively charged ions that tend to be soluble in biological fluids. It has higher affinity for groups containing sulfur, such as sulfhydryl (SH), than for ones containing oxygen.17 Lead forms covalent bonds, causing changes in the properties of sulfhydryl-containing enzymes, such as solubility, dissociation, the relative affinity to receptors, distribution and excretion.17 Lead toxicity affects several organ systems, including nervous, hematopoietic, renal, endocrine and skeletal systems.17
Melanoma is a malignant tumor which originates from melanocytes, cells that produce the pigment melanin that gives color to skin, hair and eyes. The incidence of this skin cancer has been increasing more rapidly than any other cancer type. It is one of the most aggressive malignancies in humans and is responsible for 60% to 80% of skin cancer deaths.18 This is not only because of its incidence and propensity to affect young adults, but also because of its high metastatic potential, aggressive clinical behavior, and extraordinary resistance to the currently available chemotherapeutic and immunological treatments.18 During the development of malignant melanoma, there is a complex interaction of environmental and endogenous (genetic) factors, including the deregulation of cell proliferation, the programmed cell death (apoptosis),19 and cell-cell interactions.20
Selenium and its compounds, both inorganic and organic, have recently attracted oncologists' attention after several epidemio-logical studies revealed an inverse correlation between the in-take of selenium and the incidence of cancer.21 Selenium has quite important biological and biochemical functions in organisms because of its antioxidant properties, preventing the formation of free radicals that cause DNA damage and promote tumor genesis. It also is a moderate antagonist for the toxic effect on the body of many heavy metals such as arsenic,22 cadmium,23 mercury,24–25 and lead.26 Selenium is used in methylated forms, which are less toxic and still have effects on carcinogenesis,27 and more than 90% of the experiments have used sodium selenite.28 Selenium confers protection, in part by inducing cellular free radical scavenging systems and by enhancing peroxide breakdown.29 Thus, selenium enhances the capacity of the cell to cope with oxidative stress.29 The selenoproteins (Se-P) may be useful in the prevention of cancers which are associated with persistent chronic inflammation and infection, since Se-P are presumed to be involved in alleviating the toxicity of heavy metals.30–32 Some Se-P have important enzymatic functions because they generally contain selenocysteine (SeC) in the active site, as well as cysteine (Cys) residues, indicating that it is capable of transporting selenium and bind to heavy metals.24 Thus, we believe that Se-P has three separate roles: (1) antioxidant defense; (2) a role in the transport of selenium; (3) a protective role as a natural chelator of heavy metals. In addition, the anti-tumor activity of selenium is directly related to its antioxidant activity, acting on the protection of Cys residues of reduced glutathione (GSH), which is considered to be the most important compound in the detoxification of carcinogens.24 Apoptosis and necrosis are two types of cell death that can occur due to in vivo or in vitro exposure to cadmium or lead,33 which caused an increase in lyses or necrosis. Selenium provides a significant protection against cadmium-induced apoptosis.34
Given the facts that lead is toxic by causing mainly necrosis and that selenium is an antagonist to the toxic effects of many heavy metals, the aim of this study was to investigate the potential of lead for the treatment of melanoma and the potential protective role of selenium against the toxic effects of lead. We hypothesise that lead can be used to treat melanoma, along with selenium to work against the toxic effects of lead.
C57BL/6 mice were used in this study in view of the similarity between melanoma in mice and melanoma in human and the relative ease of melanoma induction in mice compared to other animal models.35 The investigators who performed the subcutaneous injection, tumor growth measurements, flow cytometry, and the statistical analysis were not blinded to group allocation.
Seventy eight female C57BL/6 mice (Charles River Laboratories España S.A., Barcelona, Spain) between 6-8 weeks of age and weighing about 19.5 ± 2.0 g were kept under standard housing conditions (49 × 34 × 16 cm autoclavable polypropylene boxes with a wire lid and built-in feeder and water drinker, 12-hour light cycles from 7 a.m. to 7 p.m., and controlled humidity and temperature) and with food and water ad libitum. The animals were assigned randomly into 10 treatment groups (six mice per group) and one control group (n = 18) (Table 1).
Table 1.Treatment Groups.
| PbCl2 | PbCl2 | PbCl2 | PbCl2 | PbCl2 | |
|---|---|---|---|---|---|
| 0 μmol/L | 1 μmol/L | 10 μmol/L | 50 μmol/L | 100 μmol/L | |
| Na2SeO3 | 18* | 6† | 6† | 6† | 6† |
| 0 μmol/L | |||||
| Na2SeO3 | 6‡ | ||||
| 1 μmol/L | |||||
| Na2SeO3 | 6‡ | 6§ | |||
| 10 μmol/L | |||||
| Na2SeO3 | 6‡ | ||||
| 50 μmol/L | |||||
| Na2SeO3 | 6‡ | 6§ | |||
| 100 μmol/L |
All animals were treated in accordance with the European Council Directive 2010/63/EU on the protection of animals used for scientific purposes. After the experiments, the animals were euthanized with a lethal injection of lead, since it was considered to be the way which implied the minimum pain, suffering and distress (Article 6 of the European Directive).
Sodium selenite and lead (II) chloride were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA) at four concentrations: 1, 10, 50, and 100 μmol/L. The suspensions were prepared in 500 μl of sterile phosphate-buffered saline (PBS) and resuspended with 5 × 105 melanoma cells. These suspensions were injected into the air pouch of the mice, which is described later in this section.
Melanoma cells (B16-F10 cell line) were purchased from ATCC (Manassas, Virginia 20110-2209, USA). The cells were cultivated in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies, Inc., Rockville, Maryland, USA), which was supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, Utah, USA) and contained 100 units/ml of penicillin, 100 μg/ml streptomycin, and a solution of nonessential amino acids (Life Technologies, Inc.). The cells were maintained at 37ćC in a humidified atmosphere of 5% CO2 / 95% air. This was the first step of the entire experiment.
In order to form a subcutaneous air pouch, 5 ml of sterile air was injected into the subcutaneous dorsal midline of the animals 10 days after the cell culture was started. After three days, 2.5 mL of sterile air were reinjected in order to maintain the open space. This method was adapted from previously published experiments.8,36 Four days after the first injection, the following suspensions were injected directly into the air pouch:
For Groups I and II, we used lead (II) chloride or sodium selenite of four different concentrations: 1, 10, 50, and 100 μmol/L. For Group III, the molar ratio between selenium and lead was 1:1, as referenced in some recent studies regarding the molar ratio of selenium in tissues.6
Measurements of body weight as a surrogate measure for tumor weight were made 0, 2, 5, 7, 9, and 12 days after treatment. On the 12th day, we aseptically excised, weighed, and measured the size of the tumors. For calculation of tumor volume, the following formula was used:
Tumor volume (cm3) = 0.52 (length x width x height).38
For cytometric analysis, each subcutaneous exudate sample, retrieved from the already excised tumor mass via a needle introduced in the edema, was stained with TACSTM Annexin V-FITC Apoptosis Detection Kit (R&D Systems, Minneapolis, Minnesota, USA) for 15 minutes. This product detects the externalization of phosphatidylserine in apoptotic cells using recombinant annexin V conjugated to green-fluorescent FITC dye and necrotic cells using red-fluorescent propidium iodide (PI). After treatment with both probes, apoptotic cells show green fluorescence, dead cells show red and green fluorescence, and live cells show little or no fluorescence. After staining, the tumor cells were washed twice and suspended for flow analysis by fluorescence activated cell sorting (FACS) in a medium containing propidium iodite (Sigma). Data were collected on cells selected by forward light scatter (FSC) and PI gating in a FACScan analyser (Becton Dickinson) with CellQuest software.
The statistical comparison between the data collected from experimental and control groups was performed using a one-way ANOVA. The numerical data are presented as means ± standard deviations, unless otherwise specified. Statistical significance was considered for p<0.05. All statistical analyses were performed using SPSS 14.0® for Windows.
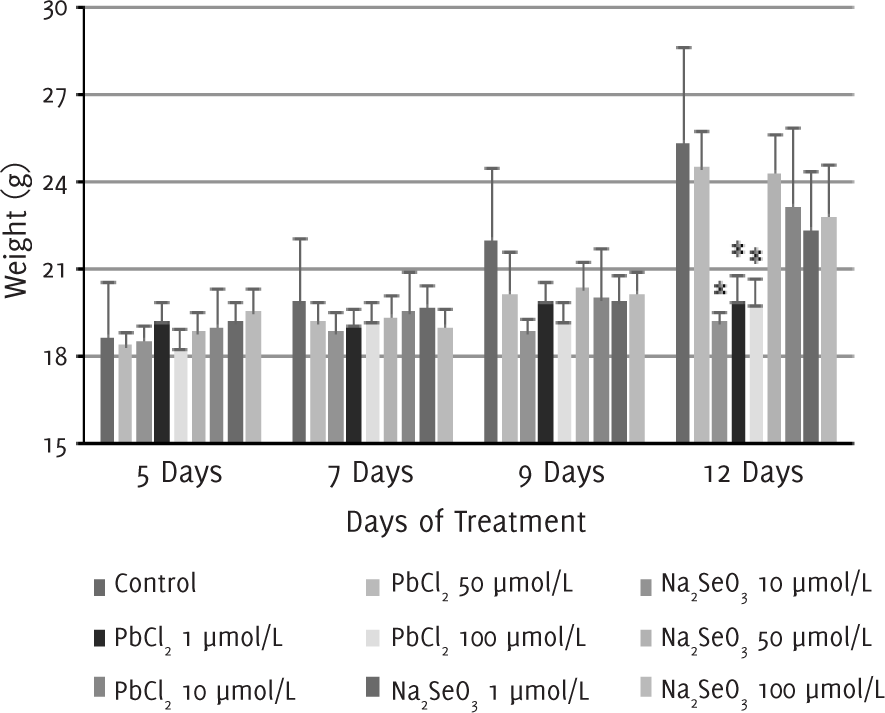
Our observations showed that 2-3 days after the injection of melanoma cells into the air pouch of the mice, tumor started to develop and a protruding mass was clearly seen under the skin around five days after injection. Between 5-12 days after treatment with lead (II) chloride, the changes in the body weight of the mice treated with lead (II) chloride 1 μmol/l were similar to that of the control group. For groups that were treated with lead (II) chloride 10, 50 and 100 μmol/l, no significant changes in body weight were observed, and the mean body weight on day 12 was significantly smaller than the control group (p<0.05) as a result. In contrast, for groups that were treated with sodium selenite 1, 10, 50 and 100 μmol/l, no significant differences were observed between the body weight of the treated mice and the control group (Figure 1).
Figure 1.Changes in Body Weight of Mice Following Lead (II) Chloride or Sodium Selenite Injections.

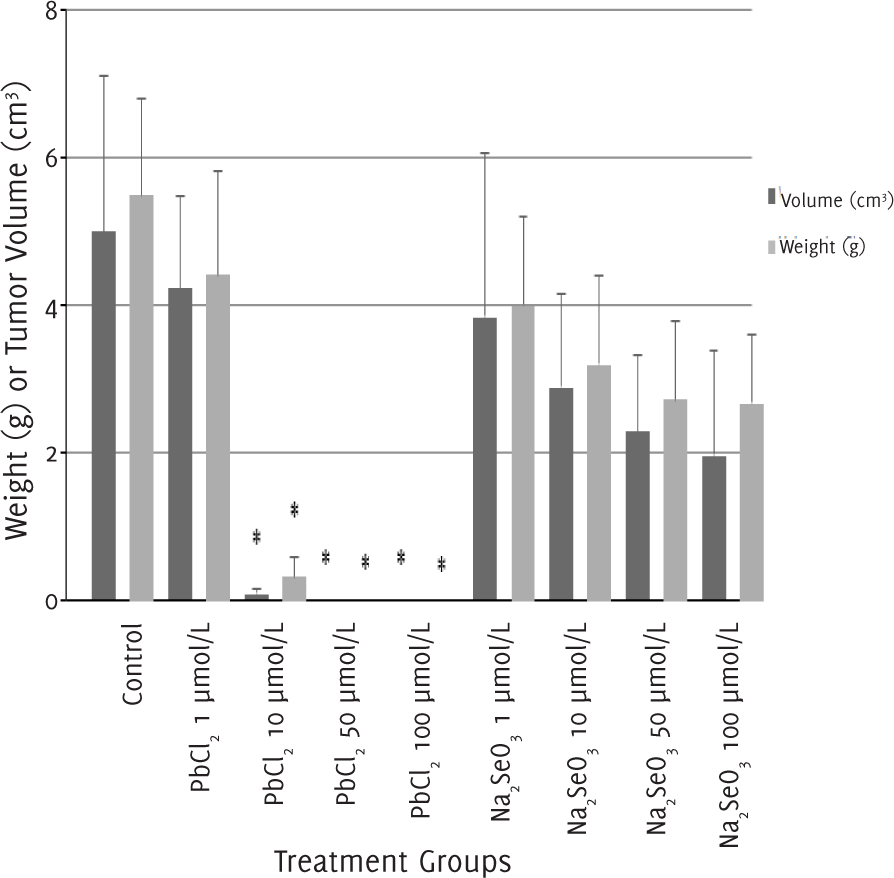
After 12 days of treatment, we excised, weighed and measured the size of the tumors (Table 2). After an injection of 1 μmol/l of lead (II) chloride, the tumor continued to develop. In contrast, mice treated with 10 μmol/l of lead (II) chloride had smaller tumor weight and tumor volume compared to the control group. For mice treated with lead (II) chloride at a concentration higher than 50 μmol/l, no tumor mass was recovered, and the differences with the control group were significant (p<0.05). The tumor weight and volume of mice treated with sodium selenite of all concentrations were similar to the control group (Figure 2).
Figure 2.Tumor Weight and Volume after 12 days of Treatment with Lead (II) Chloride or Sodium Selenite.
Tumor Weight and Volume after 12 Days of Treatment.
| Treatment groups | n | Tumor weight (g)† | Tumor volume (cm3)† |
|---|---|---|---|
| Control | 18 | 5.43 (1.31) | 4.98 (2.14) |
| PbCl2 | |||
| 1 μmol/l | 6 | 4.37 (1.38) | 4.21 (1.26) |
| 10 μmol/l | 6 | 0.32 (0.26) | 0.07 (0.07) |
| 50 μmol/l | 6 | 0 (0) | 0 (0) |
| 100 μmol/l | 6 | 0 (0) | 0 (0) |
| Na2SeO3 | |||
| 1 μmol/l | 6 | 3.95 (1.18) | 3.84 (2.22) |
| 10 μmol/l | 6 | 3.16 (1.19) | 2.88 (1.26) |
| 50 μmol/l | 6 | 2.69 (1.07) | 2.28 (1.05) |
| 100 μmol/l | 6 | 2.65 (0.91) | 1.95 (1.43) |
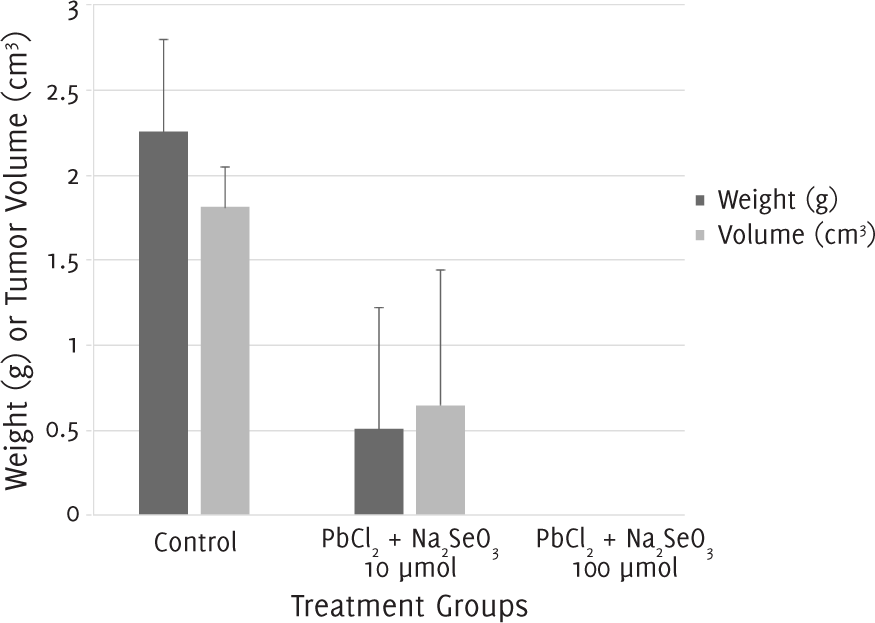
The tumor weight and volume for mice which were administered a single injection of equimolar (1:1) lead (II) chloride and sodium selenite 10 μmol/l were smaller than those of control groups. No tumor mass could be recovered from mice injected with the combination suspension at a concentration of 100 μmol/l (Figure 3).
Figure 3.Tumor Weight and Volume after 12 Days of Treatment with a Single Injection of Lead (II) Chloride (PbCl2) and Sodium Selenite (Na2SeO3).
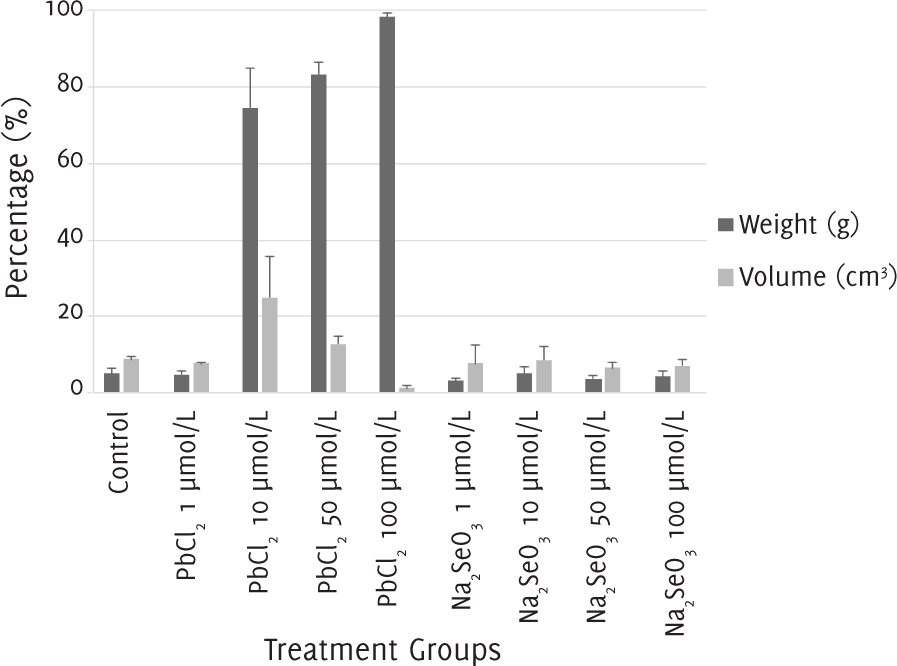
The percentage of apoptosis and necrosis in melanoma cells after 12 days of treatment with 1 μmol/l of lead (II) chloride and with 1, 10, 50 and 100 μmol/l of sodium selenite was around 10%, the same as in the control group. When the melanoma cells were treated with a concentration of 10 μmol/l of lead (II) chloride, the percentage of apoptosis was around 20% and the percentage of necrosis was nearly 80%. With a concentration of 50 μmol/l of lead (II) chloride, the percentage of apoptosis was lower at 10%, while the percentage of necrosis was higher at almost 90%. Finally, with a concentration of 100 μmol/l of lead (II) chloride, there was almost 100% of necrosis (Figure 4).
Figure 4.The Percentages of Apoptosis and Necrosis after 12 Days of Treatment with Lead (II) Chloride or Sodium Selenite.
Our study found that both the weight and total volume of tumors in mice treated with lead (II) chloride tends to be smaller with the increasing concentration of lead (II) chloride, results that are in accordance with previous studies.39 Similar findings were not observed following treatment with sodium selenite.
Lead and several inorganic lead compounds appear to have deleterious effects on skin, muscles, and the immune system.40 Lead has already been used to induce cell death in human neuroblastoma cells in previous studies.41 When neuroblastoma cells were treated with lead alone, they exhibited a significant decrease in their viability.42 Similar effects were observed in the present study: the development of melanoma appears to be negatively associated with lead (II) chloride concentration.
In contrast to findings of previous studies which suggested the potential of selenium as a cancer preventative agent and an anti-metastasis agent,43 our study did not find a significant effect of selenium on tumor development. When the tumor was treated with an equimolar injection of lead (II) chloride and sodium selenite, the weight and total volume of the tumor were smaller than the control group at a concentration of 10 μmol/l, and no tumor was recovered at a concentration of 100 μmol/l. The effects of lead (II) chloride or lead (II) chloride + sodium selenite on tumor growth were similar.
Selenium has a protective role against the development of tumors because it delays the oxidative damage in DNA and lipids as well as regulates cellular and molecular events that are essential for cell growth and carcinogenesis.44 Selenium is also considered to protect the organism in cases of poisoning with lead, mercury and cadmium.45 When melanoma cells were treated with lead (II) chloride ≥ 50 μmol/l, the percentage of cell with necrosis increased. However, when they were treated with the same concentrations of sodium selenite, no significant differences in the percentages of apoptosis and necrosis were observed between the treatment groups and the control group. Thus, our findings were in accordance with other experiments which showed that the cadmium and lead caused an increase of lyses or necrosis.33
Given the facts that chemotherapy and radiotherapy eliminate tumor cells by inducing apoptosis and that melanocytes are resistant to apoptosis,46–48 it is important to study the possibility of eradicating malignant tumor cells via necrosis induced by lead in certain concentrations. Nonetheless, it is also important to keep in mind that necrosis triggers inflammatory processes and thus it is vital to use an antagonist (such as selenium) to reduce the toxic and inflammatory effects of lead. Hence, the main goal is to make a compromise between the lead concentration and tumor cell destruction without causing severe inflammation problems as well as lead intoxications. This can be counteracted by using selenium as a lead toxicity detoxifier.
Our study findings demonstrated the potential of lead as a possible therapy for melanoma via the induction of necrosis, the accompanying inflammatory reactions of which could be counteracted by administering lead in combination with selenium, a lead antagonist. Future studies conducted using other techniques as well as full pathological studies are necessary to explore further the effects of lead and selenium on melanoma induction.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conception and design the work/idea: IS, EC. Collect data/obtaining results: IS. Analysis and interpretation of data: IS, TN, EC. Write the manuscript: IS, TN. Critical revision of the manuscript: TN, EC. Approval of the final version, Contribution of patients or study material, Obtaining financing: EC.
1. Durham TR, Snow ET. Metal ions and carcinogenesis. EXS. 2006;(96):97–130.
2. Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010 Oct;23(5):783–92.
3. Salińska A, Włostowski T, Maciak S, Łaszkiewicz-Tiszczenko B, Kozłowski P. Combined effect of dietary cadmium and benzo(a)pyrene on metallothionein induction and apoptosis in the liver and kidneys of bank voles. Biol Trace Elem Res. 2012 Jun;147(1-3):189–94.
4. Zhang C, Song N, Zeng GM, Jiang M, Zhang JC, Hu XJ, et al. Bioaccumulation of zinc, lead, copper, and cadmium from contaminated sediments by native plant species and Acrida cinerea in South China. Environ Monit Assesss. 2014 Mar;186(3):1735–45.
5. Cunha EM, Silva DP, Aguas AP. High-resolution identification of mercury in particles in mouse kidney after acute lethal exposure. Biometals. 2003 Dec;16(4):583–90.
6. Park JD, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health. 2012 Nov;45(6):344–52.
7. Benjelloun M, Tarrass F, Hachim K, Medkouri G, Benghanem MG, Ramdani B. Chronic lead poisoning: a “forgotten” cause of renal disease. Saudi J Kidney Dis Transpl. 2007 Mar;18(1):83–6.
8. Sá I, da Costa MJ Cunha EM. Lead hapatotoxicology: a study in an animal model. Toxicol Ind Health. 2012 Mar;28(2):108–13.
9. Sanín LH González-Cossío T, Romieu I, Hernández-Avila M. [Accumulation of lead in bone and its effects on health]. Salud Publica Mex. 1998 Jul-Aug;40(4):359–68. Spanish.
10. Gangoso L, Alvarez-Lloret P, Rodríguez-Navarro AA Mateo R, Hiraldo F, Donázar JA. Long-term effects of lead poisoning on bone mineralization in vultures exposed to ammunition sources. Environ Pollut. 2009 Feb;157(2):569–74.
11. Koedrith P, Seo YR. Advances in carcinogenic metal toxicity and potential molecular markers. Int J Mol Sci. 2011;12:9576–95.
12. Koedrith P, Kim H, Weon JI, Seo YR. Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int J Hyg Environ Health. 2013 Aug;216(5):587–98.
13. Khlifi R, Olmedo P, Gil F, Hammami B, Chakroun A, Rebai A, et al. Arsenic, cadmium, chromium and nickel in cancerous and healthy tissues from patients with head and neck cancer. Sci Total Environ. 2013 May 1; 452-453:58–67.
14. Huang HH, Huang JY, Lung CC, Wu CL, Ho CC, Sun YH, et al. Cell-type specificity of lung cancer associated with low-dose soil heavy metal contamination in Taiwan: an ecological study. BMC Public Health. 2013 Apr 10;13:330.
15. Carpenter RL, Jiang BH. Roles of EGFR, PI3K, AKT, and mTOR in heavy metal-induced cancer. Curr Cancer Drug Targets. 2013 Mar;13(3):252–66.
16. Goering PL. Lead-protein interactions as a basis for lead toxicity. Neurotoxicology. 1993 Summer-Fall;14(2-3):45–60.
17. Magyar JS, Weng TC, Stern CM, Dye DF, Rous BW, Payne JC, et al. Reexamination of lead(II) coordination preferences in sulfur-rich sites: implications for a critical mechanism of lead poisoning. J Am Chem Soc. 2005 Jul 6;127(26):9495–505.
18. Francis SO, Mahlberg MJ, Johnson KR, Ming ME, Dellavalle RP. Melanoma chemoprevention. J Am Acad of Dermatol. 2006 Nov;55(5):849–61.
19. Rikiishi H. Apoptotic cellular events for selenium compounds involved in cancer prevention. J Bioenerg Biomembr. 2007 Feb;39(1):91–8.
20. Bandarchi B, Jabbari CA, Vedadi A, Navab R. Molecular biology of normal melanocytes and melanoma cells. J Clin Pathol. 2013 Aug;66(8):644–8.
21. Wang X, Sun K, Tan Y, Wu S, Zhang J. Efficacy and safety of selenium nanoparticles administered intraperitoneally for the prevention of growth of cancer cells in the peritoneal cavity. Free Radic Biol Med. 2014 Jul;72:1–10.
22. Rodríguez-Sosa M, García-Montalvo EA Del Razo LM Vega L. Effect of selenomethionine supplementation in food on the excretion and toxicity of arsenic exposure in female mice. Biol Trace Elem Res. 2013 Dec;156(1-3):279–87.
23. Li JL, Jiang CY, Li S, Xu SW. Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicol Environ Saf. 2013 Oct;96:103–9.
24. Goyer RA. Perspective on low level lead toxicity. Environ Health Perspect. 1974 May;7:1.
25. Cerklewski FL, Forbes RM. Influence of dietary selenium on lead toxicity in the rat. J Nutr. 1976 Jun;106(6):778–83.
26. Li M, Gao JQ, Li XW. [Antagonistic action of selenium against the toxicity of lead]. Wei Sheng Yan Jiu. 2005 May;34(3):375–7. Chinese.
27. Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999 Sep;20(9):1657–66.
28. Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998 Nov;128(11):1845–54.
29. Reinhold U, Biltz H, Bayer W, Schmidt KH. Serum selenium levels in patients with malignant melanoma. Acta Derm Venereol. 1989;69(2):132–6.
30. Whanger PD. Selenium in the treatment of heavy metal poisoning and chemical carcinogenesis. J Trace Elem Electrolytes Health Dis. 1992 Dec;6(4):209–21.
31. Wanger PD. Selenium and the brain: a review. Nutr Neurosci. 2001;4(2):81–97.
32. Felix K, Gerstmeier S, Kyriakopoulos A, Howard OM, Dong HF, Eckhaus M, et al. Selenium deficiency abrogates inflammation-dependent plasma cell tumors in mice. Cancer Res. 2004 Apr 15:64(8):2910–7.
33. Hernández-García A, Romero D, Gómez-Ramírez P, María-Mojica P, Martínez-López E, García-Fernández AJ. In vitro evaluation of cell death induced by cadmium, lead and their binary mixtures on erythrocytes of Common buzzard (Buteo buteo). Toxicol In Vitro. 2014 Mar;28(2):300–6.
34. Zhou YJ, Zhang SP, Liu CW, Cai YQ. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLCPK(1) cells. Toxicol In Vitro. 2009 Mar;23(2):288–94.
35. Namdar A, Mirzaei HR, Jadidi-Niaragh F, Ashourpour M, Ajami M, Hadjati J, et al. Multiple low doses of 5-fluorouracil diminishes immunosuppression by myeloid derived suppressor cells in murine melanoma model. Iran J Immunol. 2015 Sep;12(3):176–87.
36. Costa MM, Águas AP. Inflammatory granulocytes decrease subcutaneous growth of melanoma in mice. Inflammation. 2004 Dec;28(6):355–7.
37. Drash G, Mail der S, Schlosser C, Roider G. Content of non-mercury-associated selenium in human tissues. Biol Trace Elem Res. 2000 Dec;77(3):219–30.
38. Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–54.
39. Rafales LS, Bornschein RL, Michaelson IA, Loch RK, Barker GF. Drug Induced activity in lead-exposed mice. Pharmaco Biochem Behav. 1979 Jan;10(1):95–104
40. World Health Organization. Environmental Health Criteria 165: inorganic lead. Geneva: World Health Organization; 1995.
41. Chetty CS, Vemuri MC, Campbell K, Suresh C. Lead-induced cell death of human neuroblastoma cells involves GSH deprivation. Cell Mol Biol Lett. 2005;10(3):413–23.
42. Suresh C, Johnson J, Mohan R, Chetty CS. Synergistic effects of amyloid peptides and lead on human neuroblastoma cells. Cell Mol Biol Lett. 2012 Sep;17(3):408–21.
43. Chen YC, Prabhu KS, Mastro AM. Is selenium a potential treatment for cancer metastasis? Nutrients. 2013 Apr 8;5(4):1149–68.
44. Agency for Toxic Substances and Disease Registry (ATSDR), 2003.
45. Umińska R. [Selenium in human environment]. Rocz Panstw Zakl Hig. 1990;41(1-2):25–34. Polish.
46. Tiezzi DG, De Andrade JM Cândido dos Reis FJ, Marana HR, Ribeiro-Silva A, Tiezzi MG, et al. Apoptosis induced by neoadjuvant chemotherapy in breast cancer. Pathology. 2006 Feb;38(1):21–7.
47. Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer. 1999 Mar;6(1):41–4.
48. Rass K, Hassel JC. Chemotherapeutics, chemoresistance and the management of melanoma. G Ital Dermatol Venereol. 2009 Feb;144(1):61–78.
Isabel Sá, 1 University of Vigo, Spain.
Tânia Nogueira, 1 University of Vigo, Spain.
Elisabete Cunha, 2 Faculty of Medicine University of Porto, Portugal.
About the Author: Elisabete Cunha is currently a firstyear intern at Hospital de São João in Porto, Portugal.
Correspondence: Isabel Sá. Address: Lagoas s/n, 36310 Vigo, Pontevedra, Spain. Email:maria.isabel.sa@hotmail.com
Cite as: Sá I, Nogueira T, Cunha E. The Effects of Lead and Selenium on Melanoma Induction. Int J Med Students. 2015 Apr-Aug;3(2):83-7.
Copyright © 2015 Isabel Sá, Tânia Nogueira, Elisabete Cunha
International Journal of Medical Students, VOLUME 3, NUMBER 2, August 2015